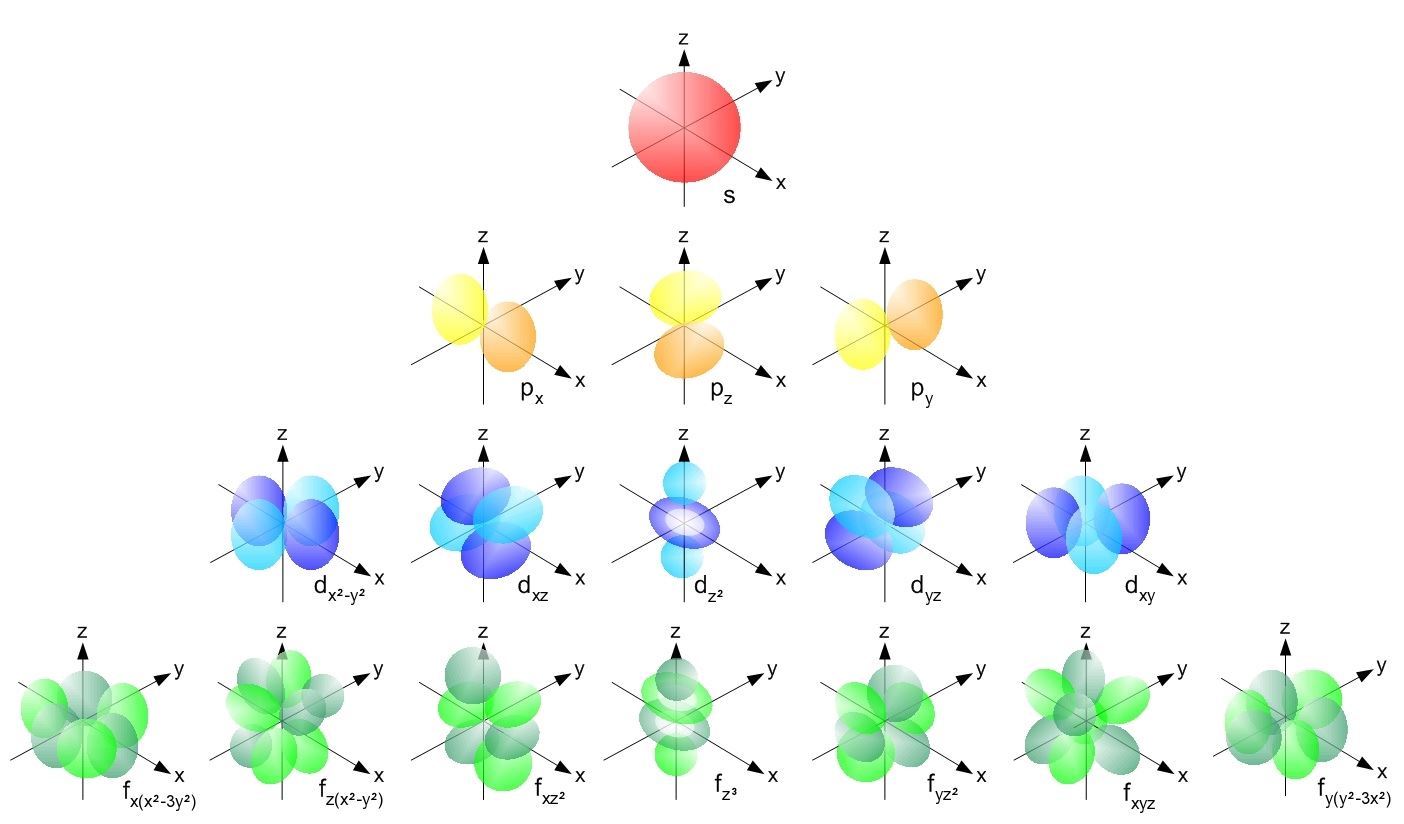
Shell Definition Quantum . Each allowed electron orbit is. The lowest energy shell has n = 1. The arrangement of electrons in an atom is called the electronconfiguration. Other shells have higher energy. The shells of an atom can be thought of concentric circles radiating out from. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. The principal quantum number, \(n\), designates the principal electron shell. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Because n describes the most probable distance of the electrons from the nucleus, the. Another name for the principal quantum number is the shell number. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. These shells are defined by the principal quantum number, given the symbol 'n'. Within a shell (same n n), all electrons that share the same l l (the angular.
from byjus.com
All electrons that have the same value for n n (the principle quantum number) are in the same shell. Other shells have higher energy. The principal quantum number, \(n\), designates the principal electron shell. Within a shell (same n n), all electrons that share the same l l (the angular. These shells are defined by the principal quantum number, given the symbol 'n'. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Another name for the principal quantum number is the shell number. The arrangement of electrons in an atom is called the electronconfiguration. Each allowed electron orbit is. Because n describes the most probable distance of the electrons from the nucleus, the.
Quantum Numbers (Principal, Azimuthal, and Spin) Definition
Shell Definition Quantum Another name for the principal quantum number is the shell number. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Other shells have higher energy. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. The arrangement of electrons in an atom is called the electronconfiguration. The lowest energy shell has n = 1. Within a shell (same n n), all electrons that share the same l l (the angular. The shells of an atom can be thought of concentric circles radiating out from. Because n describes the most probable distance of the electrons from the nucleus, the. The principal quantum number, \(n\), designates the principal electron shell. Another name for the principal quantum number is the shell number. These shells are defined by the principal quantum number, given the symbol 'n'. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Each allowed electron orbit is. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons.
From www.thoughtco.com
Subshell Definition for Electrons Shell Definition Quantum Within a shell (same n n), all electrons that share the same l l (the angular. Each allowed electron orbit is. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. Another name for. Shell Definition Quantum.
From www.youtube.com
Quantum numbers Electronic structure of atoms Chemistry Khan Shell Definition Quantum Other shells have higher energy. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. The arrangement of electrons in an atom is called the electronconfiguration. The principal quantum number, \(n\), designates the principal. Shell Definition Quantum.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.1.6 Electronic Structure翰林国际教育 Shell Definition Quantum All electrons that have the same value for n n (the principle quantum number) are in the same shell. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. These shells are defined by the principal quantum number, given the symbol 'n'. Within a shell (same n n), all electrons that share the same l l (the. Shell Definition Quantum.
From bmp-level.blogspot.com
What Is An Electron Shell Definition bmplevel Shell Definition Quantum Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The lowest energy shell has n = 1. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Each allowed electron orbit is. These shells are defined by the principal quantum number, given the symbol. Shell Definition Quantum.
From www.britannica.com
Shell atomic model Description, Configuration, Chemistry, Proposed By Shell Definition Quantum Within a shell (same n n), all electrons that share the same l l (the angular. Another name for the principal quantum number is the shell number. These shells are defined by the principal quantum number, given the symbol 'n'. The arrangement of electrons in an atom is called the electronconfiguration. The lowest energy shell has n = 1. The. Shell Definition Quantum.
From pediaa.com
Difference Between Shell Subshell and Orbital Definition, Structure Shell Definition Quantum Other shells have higher energy. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The principal quantum number, \(n\), designates the principal electron shell. The arrangement of electrons in an atom is called the. Shell Definition Quantum.
From www.breakingatom.com
Quantum particle Definition Shell Definition Quantum The shells of an atom can be thought of concentric circles radiating out from. The lowest energy shell has n = 1. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Each allowed electron orbit is. Another name for the principal quantum number is the shell number. The arrangement of electrons in an atom is called. Shell Definition Quantum.
From www.youtube.com
Relationship of Quantum shells, Subshells, Orbitals & Electrons Shell Definition Quantum Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. The arrangement of electrons in an atom is called the electronconfiguration. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. The principal quantum number, \(n\), designates the principal electron shell. Another name for. Shell Definition Quantum.
From brainly.in
differentiate between shell subshell and orbitals with the help of Shell Definition Quantum Because n describes the most probable distance of the electrons from the nucleus, the. The shells of an atom can be thought of concentric circles radiating out from. Another name for the principal quantum number is the shell number. The principal quantum number, \(n\), designates the principal electron shell. Within a shell (same n n), all electrons that share the. Shell Definition Quantum.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Shell Definition Quantum Within a shell (same n n), all electrons that share the same l l (the angular. Another name for the principal quantum number is the shell number. These shells are defined by the principal quantum number, given the symbol 'n'. Each allowed electron orbit is. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. Other. Shell Definition Quantum.
From www.researchgate.net
The buildup of electron shells in terms of quantum numbers 1930 Shell Definition Quantum The shells of an atom can be thought of concentric circles radiating out from. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. The lowest energy shell has n = 1. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. Each allowed. Shell Definition Quantum.
From www.reddit.com
ELI5 What is a shell, and orbital, and a suborbital in an atom? r Shell Definition Quantum Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Each allowed electron orbit is. The arrangement of electrons in an atom is called the electronconfiguration. Because n describes the most probable distance of the electrons from the nucleus, the. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry. Shell Definition Quantum.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science Shell Definition Quantum Each allowed electron orbit is. Because n describes the most probable distance of the electrons from the nucleus, the. The arrangement of electrons in an atom is called the electronconfiguration. The principal quantum number, \(n\), designates the principal electron shell. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The shells of an atom can be. Shell Definition Quantum.
From www.youtube.com
Electron Shells 🐚 Principal Quantum Number (n) Atoms, Protons Shell Definition Quantum The lowest energy shell has n = 1. All electrons that have the same value for n n (the principle quantum number) are in the same shell. These shells are defined by the principal quantum number, given the symbol 'n'. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. Within a shell (same n n),. Shell Definition Quantum.
From www.linstitute.net
IB DP Chemistry HL复习笔记2.1.6 Energy Levels & Sublevels翰林国际教育 Shell Definition Quantum The principal quantum number, \(n\), designates the principal electron shell. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. The lowest energy shell has n = 1. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. These shells are defined by the. Shell Definition Quantum.
From education-portal.com
Quantum Number Definition & Example Video & Lesson Shell Definition Quantum Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. The principal quantum number, \(n\), designates the principal electron shell. All electrons that have the same value for n n (the principle quantum number) are in the same shell. These shells are defined by the principal quantum number,. Shell Definition Quantum.
From www.youtube.com
AP Ch 7 Orbitals, Shells & Quantum Numbers YouTube Shell Definition Quantum All electrons that have the same value for n n (the principle quantum number) are in the same shell. The shells of an atom can be thought of concentric circles radiating out from. These shells are defined by the principal quantum number, given the symbol 'n'. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written. Shell Definition Quantum.
From www.youtube.com
3 1 3 Quantum numbers, orbitals, subshells and shells CV YouTube Shell Definition Quantum The arrangement of electrons in an atom is called the electronconfiguration. The shells of an atom can be thought of concentric circles radiating out from. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Other shells have higher energy. Because n describes the most probable distance of the electrons from. Shell Definition Quantum.
From www.allaboutcircuits.com
Quantum Physics Solidstate Device Theory Electronics Textbook Shell Definition Quantum Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. All electrons that have the same value for n n (the principle quantum number) are in the same shell. The lowest energy shell has n = 1. Electrons are arranged around the nucleus in principal energy levels or. Shell Definition Quantum.
From brainly.in
draw a diagram of bohrs model of an atom showing four energy shells Shell Definition Quantum Another name for the principal quantum number is the shell number. The principal quantum number, \(n\), designates the principal electron shell. All electrons that have the same value for n n (the principle quantum number) are in the same shell. The lowest energy shell has n = 1. The shells of an atom can be thought of concentric circles radiating. Shell Definition Quantum.
From socratic.org
What is the difference between electron shells and electron orbitals Shell Definition Quantum These shells are defined by the principal quantum number, given the symbol 'n'. The shells of an atom can be thought of concentric circles radiating out from. The arrangement of electrons in an atom is called the electronconfiguration. Each allowed electron orbit is. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry. Shell Definition Quantum.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts Shell Definition Quantum The shells of an atom can be thought of concentric circles radiating out from. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. Because n describes the most probable distance of the electrons from the nucleus, the. All electrons that have the same value for n n (the principle quantum number) are in the same. Shell Definition Quantum.
From slideplayer.com
Chapter 2 Atomic Structure ppt download Shell Definition Quantum Other shells have higher energy. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The shells of an atom can be thought of concentric circles radiating out from. Because n describes the most probable distance of the electrons from the nucleus, the. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written. Shell Definition Quantum.
From www.slideserve.com
PPT The Bohr Model of the Hydrogen Atom PowerPoint Presentation ID Shell Definition Quantum Each allowed electron orbit is. These shells are defined by the principal quantum number, given the symbol 'n'. The arrangement of electrons in an atom is called the electronconfiguration. Another name for the principal quantum number is the shell number. Other shells have higher energy. Because n describes the most probable distance of the electrons from the nucleus, the. Electrons. Shell Definition Quantum.
From www.britannica.com
Sorbital physics Britannica Shell Definition Quantum The shells of an atom can be thought of concentric circles radiating out from. Other shells have higher energy. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Each allowed electron orbit is. The arrangement of electrons in an atom is called the electronconfiguration. Within a shell (same n n), all electrons that share the same. Shell Definition Quantum.
From www.slideserve.com
PPT Key Terms Chapter 7 PowerPoint Presentation, free download ID Shell Definition Quantum Another name for the principal quantum number is the shell number. Within a shell (same n n), all electrons that share the same l l (the angular. Because n describes the most probable distance of the electrons from the nucleus, the. The principal quantum number, \(n\), designates the principal electron shell. These shells are defined by the principal quantum number,. Shell Definition Quantum.
From www.numerade.com
SOLVEDWhich quantum number defines a shell? Which quantum numbers Shell Definition Quantum Because n describes the most probable distance of the electrons from the nucleus, the. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Electrons are arranged around the nucleus in principal energy levels or principal quantum shells. Within a shell (same n n), all electrons that share the same l l (the angular. Revision notes on. Shell Definition Quantum.
From chem1.com
The quantum atom Shell Definition Quantum The shells of an atom can be thought of concentric circles radiating out from. These shells are defined by the principal quantum number, given the symbol 'n'. Because n describes the most probable distance of the electrons from the nucleus, the. Another name for the principal quantum number is the shell number. Electrons are arranged around the nucleus in principal. Shell Definition Quantum.
From www.britannica.com
Electron shell Definition & Facts Britannica Shell Definition Quantum These shells are defined by the principal quantum number, given the symbol 'n'. The shells of an atom can be thought of concentric circles radiating out from. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Other shells have higher energy. Each allowed electron orbit is. The. Shell Definition Quantum.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Shell Definition Quantum Each allowed electron orbit is. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. All electrons that have the same value for n n (the principle quantum number) are in the same shell. The principal quantum number, \(n\), designates the principal electron shell. The arrangement of electrons. Shell Definition Quantum.
From www.thestudentroom.co.uk
What exactly is a quantum shell? The Student Room Shell Definition Quantum Within a shell (same n n), all electrons that share the same l l (the angular. Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. These shells are defined by the principal quantum number,. Shell Definition Quantum.
From slideplayer.com
The Quantum View of the Atom ppt download Shell Definition Quantum Revision notes on 1.2.2 quantum shells for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. Within a shell (same n n), all electrons that share the same l l (the angular. Another name for the principal quantum number is the shell number. Because n describes the most probable distance of the electrons from. Shell Definition Quantum.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry Shell Definition Quantum These shells are defined by the principal quantum number, given the symbol 'n'. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The principal quantum number, \(n\), designates the principal electron shell. Each allowed electron orbit is. The lowest energy shell has n = 1. All electrons that have the same value for n n (the. Shell Definition Quantum.
From www.slideshare.net
Models of the Atom Shell Definition Quantum Because n describes the most probable distance of the electrons from the nucleus, the. Each allowed electron orbit is. Other shells have higher energy. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The arrangement of electrons in an atom is called the electronconfiguration. The principal quantum number, \(n\), designates the principal electron shell. Revision notes. Shell Definition Quantum.
From ar.inspiredpencil.com
Electron Orbital Shells Shell Definition Quantum The shells of an atom can be thought of concentric circles radiating out from. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Each allowed electron orbit is. Because n describes the most probable distance of the electrons from the nucleus, the. Other shells have higher energy. Within a shell. Shell Definition Quantum.