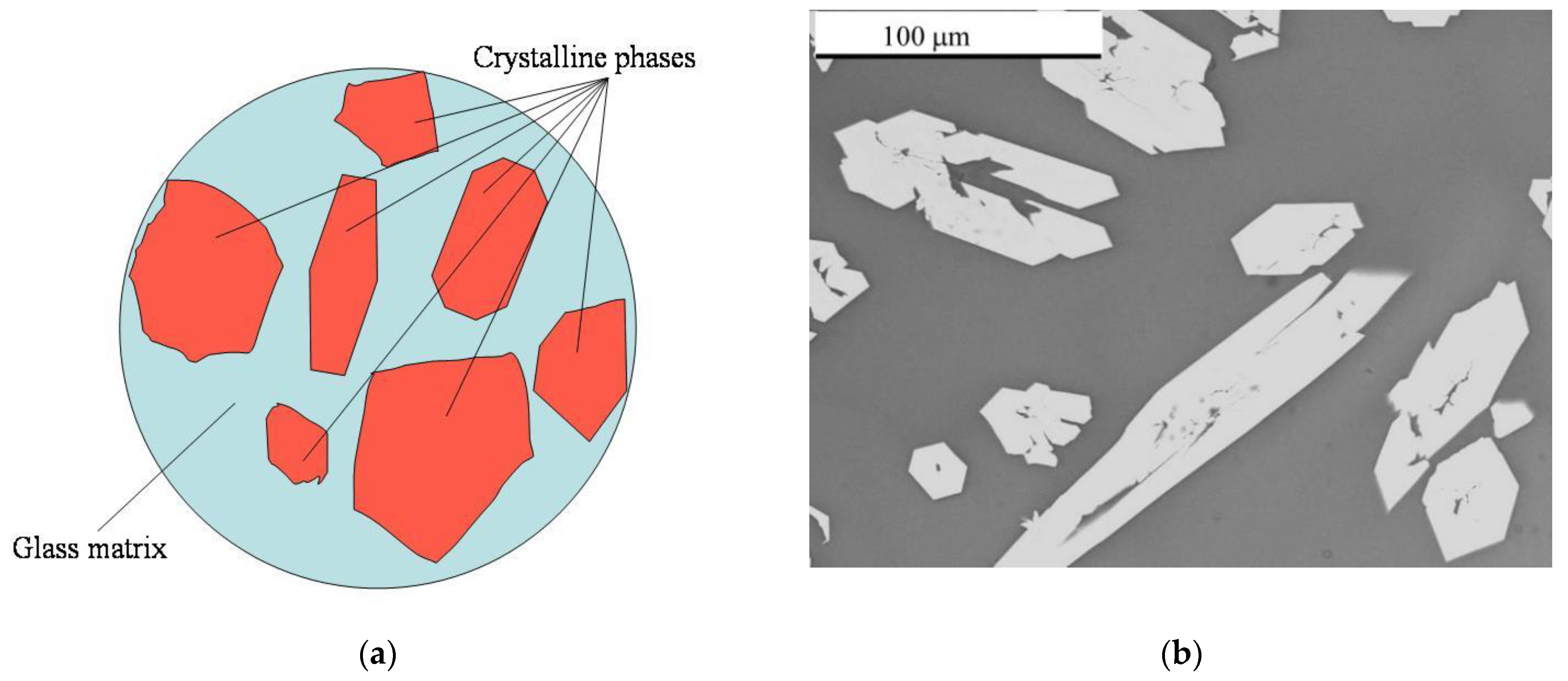
Glass Example Of Crystalline . A polycrystal is composed of many microscopic crystals (called crystallites or grains); Many materials can be made to exist as glasses. Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no. This is a heavy glass with a. Hard candies, for example, consist primarily. The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Ordinary solids, by contrast, have regular crystalline structures. The difference is illustrated in figure 1. Some substances form crystalline solids consisting of particles in a very organized structure;
from encyclopedia.pub
Hard candies, for example, consist primarily. Ordinary solids, by contrast, have regular crystalline structures. The difference is illustrated in figure 1. The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Some substances form crystalline solids consisting of particles in a very organized structure; Many materials can be made to exist as glasses. A polycrystal is composed of many microscopic crystals (called crystallites or grains); Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. This is a heavy glass with a. Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no.
Glass Crystalline Materials Encyclopedia MDPI
Glass Example Of Crystalline Some substances form crystalline solids consisting of particles in a very organized structure; A polycrystal is composed of many microscopic crystals (called crystallites or grains); The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Many materials can be made to exist as glasses. Hard candies, for example, consist primarily. Some substances form crystalline solids consisting of particles in a very organized structure; Ordinary solids, by contrast, have regular crystalline structures. The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. The difference is illustrated in figure 1. This is a heavy glass with a. Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no.
From www.friendsofglass.com
What is the difference between glass and crystal? Friends of Glass Glass Example Of Crystalline Ordinary solids, by contrast, have regular crystalline structures. Many materials can be made to exist as glasses. Hard candies, for example, consist primarily. The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. The difference is illustrated in figure 1. A polycrystal is composed of many. Glass Example Of Crystalline.
From www.nathanallan.com
Crystalline Clear Nathan Allan Glass Studios Glass Example Of Crystalline A polycrystal is composed of many microscopic crystals (called crystallites or grains); Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Some substances form crystalline solids consisting of particles in a very organized structure; This is a heavy glass with a. Hard candies, for example, consist primarily. The difference is illustrated in figure 1. Ordinary solids,. Glass Example Of Crystalline.
From www.youtube.com
Crystalline and Amorphous Solids YouTube Glass Example Of Crystalline The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. This is a heavy glass with a. The difference is illustrated in figure 1. Some substances form crystalline solids consisting of particles in a very organized structure; Ordinary solids, by contrast, have regular crystalline structures. A polycrystal is composed of many microscopic crystals. Glass Example Of Crystalline.
From www.nathanallan.com
Crystalline Series Thick Glass Glass Example Of Crystalline Some substances form crystalline solids consisting of particles in a very organized structure; This is a heavy glass with a. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no.. Glass Example Of Crystalline.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Glass Example Of Crystalline The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Many materials can be made to exist as glasses. Hard candies, for example, consist primarily. Generally, a glass exists in a structurally metastable. Glass Example Of Crystalline.
From www.nathanallan.com
Thick Glass Crystalline Clear designed and made by Nathan Allan Glass Example Of Crystalline Ordinary solids, by contrast, have regular crystalline structures. A polycrystal is composed of many microscopic crystals (called crystallites or grains); The difference is illustrated in figure 1. Some substances form crystalline solids consisting of particles in a very organized structure; The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Hard candies, for. Glass Example Of Crystalline.
From learn.careers360.com
Show the structure of crystalline, polycrystalline and amorphous Glass Example Of Crystalline Many materials can be made to exist as glasses. The difference is illustrated in figure 1. Hard candies, for example, consist primarily. A polycrystal is composed of many microscopic crystals (called crystallites or grains); Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Some substances form crystalline solids consisting of particles in a very organized structure;. Glass Example Of Crystalline.
From encyclopedia.pub
Glass Crystalline Materials Encyclopedia MDPI Glass Example Of Crystalline The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Hard candies, for example, consist primarily. Ordinary solids, by contrast, have regular crystalline structures. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. The main types of crystalline solids are ionic solids,. Glass Example Of Crystalline.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Glass Example Of Crystalline Ordinary solids, by contrast, have regular crystalline structures. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Hard candies, for example, consist primarily. Some substances form crystalline solids consisting of particles in a very organized structure; The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting. Glass Example Of Crystalline.
From www.benbest.com
LESSONS FOR CRYONICS FROM METALLURGY AND CERAMICS Glass Example Of Crystalline Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no. Ordinary solids, by contrast, have regular crystalline structures. Some substances form crystalline solids consisting of particles in a very organized structure; A polycrystal is composed of many microscopic crystals (called crystallites or grains);. Glass Example Of Crystalline.
From www.pinterest.com
Ideal Glass Would Explain Why Glass Exists at All Quanta Magazine Glass Example Of Crystalline This is a heavy glass with a. Hard candies, for example, consist primarily. The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Many materials can be made to exist as glasses. The difference is illustrated in figure 1. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. A. Glass Example Of Crystalline.
From psiberg.com
Crystal Structures Types with Explanation PSIBERG Glass Example Of Crystalline The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. A polycrystal is composed of many microscopic crystals (called crystallites or grains); Ordinary solids, by contrast, have regular crystalline structures. The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is. Glass Example Of Crystalline.
From chem.libretexts.org
12.1 Crystalline and Amorphous Solids Chemistry LibreTexts Glass Example Of Crystalline Some substances form crystalline solids consisting of particles in a very organized structure; The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Hard candies, for example, consist primarily. The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids.. Glass Example Of Crystalline.
From safesilica.eu
Crystalline Silica The Science Safe Silica Glass Example Of Crystalline The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Some substances form crystalline solids consisting of particles in a very organized structure; The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. A polycrystal is composed of many. Glass Example Of Crystalline.
From www.aniwaa.com
Thermoplastics for AM SemiCrystalline vs Amorphous Glass Example Of Crystalline Hard candies, for example, consist primarily. The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no. Many materials can be made to exist as glasses. Ordinary. Glass Example Of Crystalline.
From exokbmzkz.blob.core.windows.net
Crystalline Atoms Definition at Priscilla Anderson blog Glass Example Of Crystalline The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. This is a heavy glass with a. Many materials can be made to exist as glasses. Ordinary solids, by contrast, have regular crystalline. Glass Example Of Crystalline.
From www.nathanallan.com
Crystalline Granite Textured Glass created by Nathan Allan Glass Studios Glass Example Of Crystalline Many materials can be made to exist as glasses. The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. The difference is illustrated in figure 1. The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. This is a. Glass Example Of Crystalline.
From askfilo.com
Classification of crystalline solids Crystalline solids are classified in.. Glass Example Of Crystalline The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. The difference is illustrated in figure 1. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although. Glass Example Of Crystalline.
From www.mpg.de
From thin silicate films to the atomic structure of glass Max Planck Glass Example Of Crystalline The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. A polycrystal is composed of many microscopic crystals (called crystallites or grains); Hard candies, for example, consist primarily. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. This is a heavy glass with a. Some substances form crystalline solids. Glass Example Of Crystalline.
From www.nathanallan.com
Crystalline Series Nathan Allan Glass Studios Glass Example Of Crystalline The difference is illustrated in figure 1. The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Ordinary solids, by contrast, have regular crystalline structures. Many materials can be made to exist as glasses. The main types of crystalline solids are ionic solids, metallic solids, covalent. Glass Example Of Crystalline.
From collegedunia.com
Amorphous Solid Definition, Properties & Examples Glass Example Of Crystalline Ordinary solids, by contrast, have regular crystalline structures. The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Some substances form crystalline solids consisting of particles in a very organized structure; A polycrystal. Glass Example Of Crystalline.
From mungfali.com
Atomic Structure Of Glass Glass Example Of Crystalline Some substances form crystalline solids consisting of particles in a very organized structure; Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. A polycrystal is composed of many microscopic crystals (called crystallites or grains); Many materials can be made to exist as glasses. Hard candies, for example, consist primarily. Generally, a glass exists in a structurally. Glass Example Of Crystalline.
From www.researchgate.net
Example of a typical microstructure of a glassceramic obtained by bulk Glass Example Of Crystalline Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no. Ordinary solids, by contrast, have regular crystalline structures. The difference is illustrated in figure 1. Many materials can be made. Glass Example Of Crystalline.
From eduinput.com
Classification of Solids Crystalline Solids, Amorphous Solids, and Glass Example Of Crystalline The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. A polycrystal is composed of many microscopic crystals (called crystallites or grains); The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Some substances form crystalline solids consisting of. Glass Example Of Crystalline.
From eduinput.com
Crystalline solids Properties, types, examples use Glass Example Of Crystalline Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. A polycrystal is composed of many microscopic crystals (called crystallites or grains); Some substances form crystalline solids consisting of particles. Glass Example Of Crystalline.
From opengeology.org
13 Crystal Structures Mineralogy Glass Example Of Crystalline The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. The difference is illustrated in figure 1. Many materials can be made to exist as glasses. A polycrystal is composed of many microscopic crystals (called crystallites or grains); This is a heavy glass with a. Hard. Glass Example Of Crystalline.
From www.youtube.com
Crystalline Ceramics Technique Project YouTube Glass Example Of Crystalline This is a heavy glass with a. The difference is illustrated in figure 1. A polycrystal is composed of many microscopic crystals (called crystallites or grains); Some substances form crystalline solids consisting of particles in a very organized structure; The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point,. Glass Example Of Crystalline.
From www.nathanallan.com
Crystalline Granite designed and made by Nathan Allan Glass Example Of Crystalline The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Many materials can be made to exist as glasses. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Hard candies, for example, consist primarily. A polycrystal is composed of many microscopic crystals. Glass Example Of Crystalline.
From slideplayer.com
What is a mineral? "A mineral is a naturally occurring, homogeneous Glass Example Of Crystalline Some substances form crystalline solids consisting of particles in a very organized structure; A polycrystal is composed of many microscopic crystals (called crystallites or grains); Many materials can be made to exist as glasses. Hard candies, for example, consist primarily. The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting. Glass Example Of Crystalline.
From webmis.highland.cc.il.us
Crystalline and Amorphous Solids Glass Example Of Crystalline A polycrystal is composed of many microscopic crystals (called crystallites or grains); Ordinary solids, by contrast, have regular crystalline structures. Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no. This is a heavy glass with a. Many materials can be made to. Glass Example Of Crystalline.
From present5.com
Physical Properties Glass and Soil and Impression Analysis Glass Example Of Crystalline Ordinary solids, by contrast, have regular crystalline structures. The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Many materials can be made to exist as glasses. A polycrystal is composed of many microscopic crystals (called crystallites or grains); This. Glass Example Of Crystalline.
From chem.libretexts.org
Crystalline Solid Structures Chemistry LibreTexts Glass Example Of Crystalline Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no. Ordinary solids, by contrast, have regular crystalline structures. Some substances form crystalline solids consisting of particles in a very organized structure; Hard candies, for example, consist primarily. Many materials can be made to. Glass Example Of Crystalline.
From www.cmog.org
All About Glass Corning Museum of Glass Glass Example Of Crystalline The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no. Many materials can be made to exist as glasses. This is a heavy glass with a.. Glass Example Of Crystalline.
From www.researchgate.net
Schematic of structure of SiO2(A) glass and (B) crystal [34 Glass Example Of Crystalline The glass transition point, \(t_g\), (temperature at which a supercooled liquid becomes a glass) is for glasses what the melting point, \(t_m\), is for. Many materials can be made to exist as glasses. Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. This is a heavy glass with a. Hard candies, for example, consist primarily. The. Glass Example Of Crystalline.
From www.nathanallan.com
Crystalline Clear designed and made by Nathan Allan Glass Example Of Crystalline Others form amorphous (noncrystalline) solids with an internal structure that is not ordered. Ordinary solids, by contrast, have regular crystalline structures. A polycrystal is composed of many microscopic crystals (called crystallites or grains); This is a heavy glass with a. Many materials can be made to exist as glasses. Generally, a glass exists in a structurally metastable state with respect. Glass Example Of Crystalline.