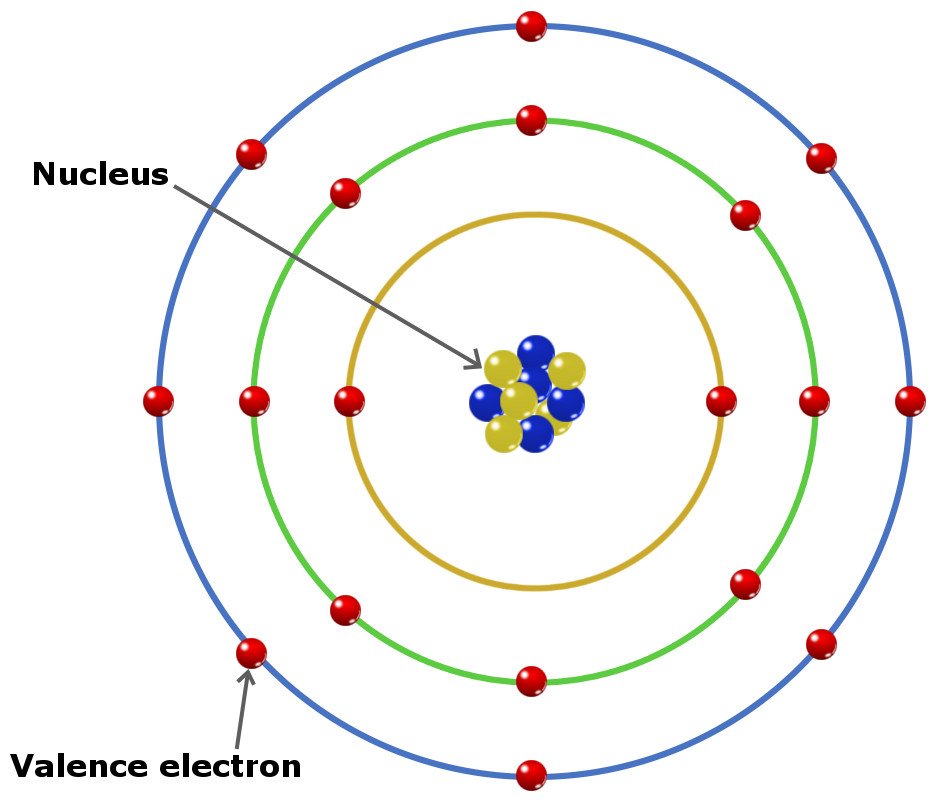
How Do Electron Shells Work . The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. As we discussed previously, this does not refer to. It is a group of atomic orbitals with the same value of the principal. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. What is an electron shell with a model and diagram. Learn how many electrons are found in each shell with the rule, order, and. Electrons in atoms occupy energy levels, also called electron shells, outside the. These shells are defined by the principal quantum number, given the symbol 'n'. As per the bohr model, electrons are arranged in shells. An electron shell is the outside part of an atom around the atomic nucleus. The energy levels for the electrons in an atom are often referred to as electron shells. Different shells can hold different maximum numbers of electrons.
from www.expii.com
These shells are defined by the principal quantum number, given the symbol 'n'. Different shells can hold different maximum numbers of electrons. It is a group of atomic orbitals with the same value of the principal. Electrons in atoms occupy energy levels, also called electron shells, outside the. As we discussed previously, this does not refer to. As per the bohr model, electrons are arranged in shells. What is an electron shell with a model and diagram. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. Learn how many electrons are found in each shell with the rule, order, and.
Valence Electrons — Definition & Importance Expii
How Do Electron Shells Work These shells are defined by the principal quantum number, given the symbol 'n'. As per the bohr model, electrons are arranged in shells. The energy levels for the electrons in an atom are often referred to as electron shells. It is a group of atomic orbitals with the same value of the principal. Learn how many electrons are found in each shell with the rule, order, and. As we discussed previously, this does not refer to. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. What is an electron shell with a model and diagram. These shells are defined by the principal quantum number, given the symbol 'n'. Electrons in atoms occupy energy levels, also called electron shells, outside the. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. An electron shell is the outside part of an atom around the atomic nucleus. Different shells can hold different maximum numbers of electrons.
From www.britannica.com
Atom Electrons, Nucleus, Bonds Britannica How Do Electron Shells Work As we discussed previously, this does not refer to. It is a group of atomic orbitals with the same value of the principal. Electrons in atoms occupy energy levels, also called electron shells, outside the. What is an electron shell with a model and diagram. Different shells can hold different maximum numbers of electrons. Progressing from one atom to the. How Do Electron Shells Work.
From wiringguidefrosts.z19.web.core.windows.net
Electron Orbital Diagram Chart How Do Electron Shells Work These shells are defined by the principal quantum number, given the symbol 'n'. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. The energy levels for the electrons in an atom are often referred to as electron shells. It is a. How Do Electron Shells Work.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science How Do Electron Shells Work Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. As per the bohr model, electrons are arranged in shells. As we discussed previously, this does not refer to. Electrons in atoms occupy energy levels, also called electron shells, outside the. These. How Do Electron Shells Work.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements How Do Electron Shells Work Different shells can hold different maximum numbers of electrons. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. These shells are defined by the principal quantum number, given the symbol 'n'. An electron shell is the outside part of an atom. How Do Electron Shells Work.
From www.nagwa.com
Lesson Video Electron Shells Nagwa How Do Electron Shells Work The energy levels for the electrons in an atom are often referred to as electron shells. As per the bohr model, electrons are arranged in shells. An electron shell is the outside part of an atom around the atomic nucleus. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting. How Do Electron Shells Work.
From middleschoolscience.com
Patterns of the Periodic Table Finding Shells and Valence Electrons How Do Electron Shells Work Electrons in atoms occupy energy levels, also called electron shells, outside the. As we discussed previously, this does not refer to. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. Different shells can hold different maximum numbers of electrons. What is an electron shell with a model and diagram. As per the. How Do Electron Shells Work.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry How Do Electron Shells Work Electrons in atoms occupy energy levels, also called electron shells, outside the. The energy levels for the electrons in an atom are often referred to as electron shells. Learn how many electrons are found in each shell with the rule, order, and. Progressing from one atom to the next in the periodic table, the electron structure can be worked out. How Do Electron Shells Work.
From chemistrytalk.org
Electron Shells ChemTalk How Do Electron Shells Work The energy levels for the electrons in an atom are often referred to as electron shells. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. Learn how many electrons are found in each shell with the rule, order, and. It is. How Do Electron Shells Work.
From lessonfullgalleting.z21.web.core.windows.net
Structure Of Atom Bohr Model How Do Electron Shells Work What is an electron shell with a model and diagram. Different shells can hold different maximum numbers of electrons. These shells are defined by the principal quantum number, given the symbol 'n'. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. An electron shell is the outside part of an atom around. How Do Electron Shells Work.
From www.animalia-life.club
Electron Shell Diagram How Do Electron Shells Work What is an electron shell with a model and diagram. As per the bohr model, electrons are arranged in shells. An electron shell is the outside part of an atom around the atomic nucleus. Learn how many electrons are found in each shell with the rule, order, and. It is a group of atomic orbitals with the same value of. How Do Electron Shells Work.
From www.animalia-life.club
Electron Shell Diagram How Do Electron Shells Work As per the bohr model, electrons are arranged in shells. It is a group of atomic orbitals with the same value of the principal. These shells are defined by the principal quantum number, given the symbol 'n'. Electrons in atoms occupy energy levels, also called electron shells, outside the. Different shells can hold different maximum numbers of electrons. What is. How Do Electron Shells Work.
From www.expii.com
Valence Electrons — Definition & Importance Expii How Do Electron Shells Work An electron shell is the outside part of an atom around the atomic nucleus. These shells are defined by the principal quantum number, given the symbol 'n'. As we discussed previously, this does not refer to. Learn how many electrons are found in each shell with the rule, order, and. What is an electron shell with a model and diagram.. How Do Electron Shells Work.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry How Do Electron Shells Work Electrons in atoms occupy energy levels, also called electron shells, outside the. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. An electron shell is the outside part of an atom around the atomic nucleus. What is an electron shell with. How Do Electron Shells Work.
From courses.lumenlearning.com
Organization of Electrons in Atoms Introductory Chemistry How Do Electron Shells Work Electrons in atoms occupy energy levels, also called electron shells, outside the. As we discussed previously, this does not refer to. What is an electron shell with a model and diagram. These shells are defined by the principal quantum number, given the symbol 'n'. As per the bohr model, electrons are arranged in shells. Learn how many electrons are found. How Do Electron Shells Work.
From www.youtube.com
Elements, Atoms, Shells, Subshells And Orbitals YouTube How Do Electron Shells Work Electrons in atoms occupy energy levels, also called electron shells, outside the. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. As per the bohr model, electrons are arranged in shells. Different shells can hold different maximum numbers of electrons. What is an electron shell with a model and diagram. Progressing from. How Do Electron Shells Work.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind How Do Electron Shells Work As we discussed previously, this does not refer to. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. An electron shell is the outside part of an atom around the atomic nucleus. Learn how many electrons are found in each. How Do Electron Shells Work.
From biolo1100.nicerweb.com
electrons/02_06_01 How Do Electron Shells Work It is a group of atomic orbitals with the same value of the principal. What is an electron shell with a model and diagram. Learn how many electrons are found in each shell with the rule, order, and. These shells are defined by the principal quantum number, given the symbol 'n'. Electrons in atoms occupy energy levels, also called electron. How Do Electron Shells Work.
From courses.lumenlearning.com
Electrons Biology for Majors I How Do Electron Shells Work Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. These shells are defined by the principal quantum number, given the symbol 'n'. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. As per. How Do Electron Shells Work.
From slcc.pressbooks.pub
4.2 The Structure of Atoms College Biology I How Do Electron Shells Work The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. Electrons in atoms occupy energy levels, also called electron shells, outside the. The energy levels. How Do Electron Shells Work.
From www.mooramo.com
Electron Shells Mooramo How Do Electron Shells Work Electrons in atoms occupy energy levels, also called electron shells, outside the. It is a group of atomic orbitals with the same value of the principal. What is an electron shell with a model and diagram. Learn how many electrons are found in each shell with the rule, order, and. As we discussed previously, this does not refer to. These. How Do Electron Shells Work.
From www.slideserve.com
PPT Basic Chemistry PowerPoint Presentation, free download ID2614477 How Do Electron Shells Work Different shells can hold different maximum numbers of electrons. What is an electron shell with a model and diagram. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. Learn how many electrons are found in each shell with the rule, order, and. Electrons in atoms occupy energy levels, also called electron shells,. How Do Electron Shells Work.
From sciencenotes.org
List of Electron Configurations of Elements How Do Electron Shells Work Different shells can hold different maximum numbers of electrons. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. Learn how many electrons are found in each shell with the rule, order, and. Electrons in atoms occupy energy levels, also called electron shells, outside the. Progressing from one atom to the next in. How Do Electron Shells Work.
From mammothmemory.net
Electrons orbit the nucleus of an atom orbits are called she How Do Electron Shells Work The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. It is a group of atomic orbitals with the same value of the principal. Different shells can hold different maximum numbers of electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. As per the bohr model, electrons are arranged. How Do Electron Shells Work.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell How Do Electron Shells Work These shells are defined by the principal quantum number, given the symbol 'n'. Different shells can hold different maximum numbers of electrons. It is a group of atomic orbitals with the same value of the principal. Learn how many electrons are found in each shell with the rule, order, and. Electrons in atoms occupy energy levels, also called electron shells,. How Do Electron Shells Work.
From scientifictutor.org
Chem Bohr Model and Electron Shells Part 1 Scientific Tutor How Do Electron Shells Work These shells are defined by the principal quantum number, given the symbol 'n'. Learn how many electrons are found in each shell with the rule, order, and. Electrons in atoms occupy energy levels, also called electron shells, outside the. As per the bohr model, electrons are arranged in shells. Progressing from one atom to the next in the periodic table,. How Do Electron Shells Work.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements How Do Electron Shells Work As per the bohr model, electrons are arranged in shells. Different shells can hold different maximum numbers of electrons. These shells are defined by the principal quantum number, given the symbol 'n'. As we discussed previously, this does not refer to. Electrons in atoms occupy energy levels, also called electron shells, outside the. The closest orbital to the nucleus, called. How Do Electron Shells Work.
From www.alamy.com
Electron shells of the first ordinary elements of the periodic table How Do Electron Shells Work The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. The energy levels for the electrons in an atom are often referred to as electron shells. Electrons in atoms occupy energy levels, also called electron shells, outside the. Progressing from one atom to the next in the periodic table, the electron structure can. How Do Electron Shells Work.
From biologydictionary.net
Covalent Bond Biology Dictionary How Do Electron Shells Work The energy levels for the electrons in an atom are often referred to as electron shells. An electron shell is the outside part of an atom around the atomic nucleus. These shells are defined by the principal quantum number, given the symbol 'n'. Different shells can hold different maximum numbers of electrons. It is a group of atomic orbitals with. How Do Electron Shells Work.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table How Do Electron Shells Work The energy levels for the electrons in an atom are often referred to as electron shells. These shells are defined by the principal quantum number, given the symbol 'n'. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. It is a group of atomic orbitals with the same value of the principal.. How Do Electron Shells Work.
From www.youtube.com
mr i explains Electron Shells (GCSE Chemistry) YouTube How Do Electron Shells Work These shells are defined by the principal quantum number, given the symbol 'n'. The closest orbital to the nucleus, called the 1 s orbital, can hold up to two electrons. It is a group of atomic orbitals with the same value of the principal. Electrons in atoms occupy energy levels, also called electron shells, outside the. An electron shell is. How Do Electron Shells Work.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science How Do Electron Shells Work These shells are defined by the principal quantum number, given the symbol 'n'. As we discussed previously, this does not refer to. What is an electron shell with a model and diagram. The energy levels for the electrons in an atom are often referred to as electron shells. It is a group of atomic orbitals with the same value of. How Do Electron Shells Work.
From mmerevise.co.uk
Atoms Questions and Revision MME How Do Electron Shells Work These shells are defined by the principal quantum number, given the symbol 'n'. As per the bohr model, electrons are arranged in shells. Electrons in atoms occupy energy levels, also called electron shells, outside the. It is a group of atomic orbitals with the same value of the principal. What is an electron shell with a model and diagram. An. How Do Electron Shells Work.
From www.britannica.com
Electron shell Definition & Facts Britannica How Do Electron Shells Work Different shells can hold different maximum numbers of electrons. It is a group of atomic orbitals with the same value of the principal. What is an electron shell with a model and diagram. The energy levels for the electrons in an atom are often referred to as electron shells. Learn how many electrons are found in each shell with the. How Do Electron Shells Work.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics How Do Electron Shells Work The energy levels for the electrons in an atom are often referred to as electron shells. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. Electrons in atoms occupy energy levels, also called electron shells, outside the. As per the bohr. How Do Electron Shells Work.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry How Do Electron Shells Work As per the bohr model, electrons are arranged in shells. As we discussed previously, this does not refer to. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital. It is a group of atomic orbitals with the same value of the. How Do Electron Shells Work.