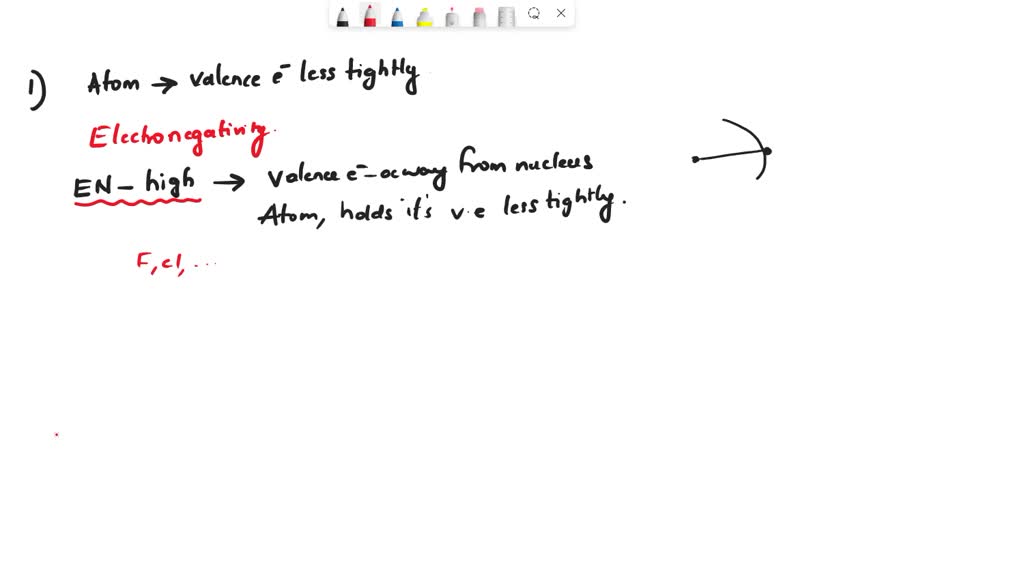
Does S Form A Positive Ion . Ionic compounds have positive ions and negative ions. Remember that ions are formed only when electrons move from one atom to another; Define ionic and molecular (covalent) compounds. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Ions form when atoms lose or gain electrons. A proton never moves from one atom to another. Ionic formulas balance the total positive and negative charges. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Conversely, ions that contain more electrons than protons have a net negative charge and are. By the end of this section, you will be able to:
from www.numerade.com
By the end of this section, you will be able to: A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Conversely, ions that contain more electrons than protons have a net negative charge and are. Remember that ions are formed only when electrons move from one atom to another; Define ionic and molecular (covalent) compounds. Ionic formulas balance the total positive and negative charges. Ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions. Ions that contain fewer electrons than protons have a net positive charge and are called cations.
SOLVED How do you determine the atom (s) that holds its valence
Does S Form A Positive Ion By the end of this section, you will be able to: Remember that ions are formed only when electrons move from one atom to another; A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Ions that contain fewer electrons than protons have a net positive charge and are called cations. By the end of this section, you will be able to: A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. Ionic compounds have positive ions and negative ions. Ionic formulas balance the total positive and negative charges. Conversely, ions that contain more electrons than protons have a net negative charge and are. A proton never moves from one atom to another. Define ionic and molecular (covalent) compounds. Ions form when atoms lose or gain electrons.
From www.slideserve.com
PPT Unit 5 Atomic Structure and the Periodic Table of Elements Does S Form A Positive Ion Ionic formulas balance the total positive and negative charges. Define ionic and molecular (covalent) compounds. Ions that contain fewer electrons than protons have a net positive charge and are called cations. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A proton. Does S Form A Positive Ion.
From www.youtube.com
What is an Ion? YouTube Does S Form A Positive Ion Ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions. Define ionic and molecular (covalent) compounds. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to. Does S Form A Positive Ion.
From studylibrarybeverly.z21.web.core.windows.net
How Do Positive And Negative Ions Form Does S Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Conversely, ions that contain more electrons than protons have a net negative charge and are. Remember that. Does S Form A Positive Ion.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Does S Form A Positive Ion Define ionic and molecular (covalent) compounds. Ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions. Ionic formulas balance the total positive and negative charges. By the end of this section, you will be able to: A proton never moves from one atom to another. Remember that ions are formed only when electrons move. Does S Form A Positive Ion.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Does S Form A Positive Ion Conversely, ions that contain more electrons than protons have a net negative charge and are. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence. Does S Form A Positive Ion.
From alevelbiology.co.uk
Ions Types, Summary, Classification & Facts Does S Form A Positive Ion Conversely, ions that contain more electrons than protons have a net negative charge and are. By the end of this section, you will be able to: A proton never moves from one atom to another. Ionic formulas balance the total positive and negative charges. Remember that ions are formed only when electrons move from one atom to another; Ionic compounds. Does S Form A Positive Ion.
From www.numerade.com
SOLVED How do you determine the atom (s) that holds its valence Does S Form A Positive Ion Define ionic and molecular (covalent) compounds. Ionic formulas balance the total positive and negative charges. Ionic compounds have positive ions and negative ions. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Ions that contain fewer electrons than protons have a net. Does S Form A Positive Ion.
From www.sliderbase.com
Formula of Ionic Compounds Does S Form A Positive Ion Define ionic and molecular (covalent) compounds. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Remember that ions are formed only when electrons move from one atom to another; Ionic compounds have positive ions and negative ions. Ions form when atoms lose or gain electrons. Ionic formulas balance the total positive and negative. Does S Form A Positive Ion.
From www.slideserve.com
PPT Ions PowerPoint Presentation, free download ID6738771 Does S Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or. Does S Form A Positive Ion.
From www.slideserve.com
PPT Chapter 8 Ionic Compounds PowerPoint Presentation ID4524293 Does S Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. Remember that ions are formed only when electrons move from one atom to another; Ions form when atoms lose or gain electrons. A cation. Does S Form A Positive Ion.
From www.slideshare.net
Ch 8 ionic compounds Does S Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. Ionic compounds have positive ions and negative ions. Define ionic and molecular (covalent) compounds. Conversely, ions that contain more electrons than protons have a. Does S Form A Positive Ion.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download Does S Form A Positive Ion A proton never moves from one atom to another. Remember that ions are formed only when electrons move from one atom to another; Ionic formulas balance the total positive and negative charges. Define ionic and molecular (covalent) compounds. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion. Does S Form A Positive Ion.
From www.slideserve.com
PPT The History of the Modern Periodic Table PowerPoint Presentation Does S Form A Positive Ion Define ionic and molecular (covalent) compounds. Ionic formulas balance the total positive and negative charges. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Ionic compounds have positive ions and negative ions. Conversely, ions that contain more electrons than protons have a net negative charge and are. Remember that ions are formed only. Does S Form A Positive Ion.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Does S Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. Ionic formulas balance the total positive and negative charges. Remember that ions are formed only when electrons move from one atom to another; A. Does S Form A Positive Ion.
From design.udlvirtual.edu.pe
What Does It Mean When An Ion Has A Positive Charge Design Talk Does S Form A Positive Ion Define ionic and molecular (covalent) compounds. Ionic compounds have positive ions and negative ions. Ionic formulas balance the total positive and negative charges. By the end of this section, you will be able to: A proton never moves from one atom to another. Ions that contain fewer electrons than protons have a net positive charge and are called cations. A. Does S Form A Positive Ion.
From www.yourdictionary.com
Ion Examples With Positive & Negative Charges YourDictionary Does S Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Define ionic and molecular (covalent) compounds. A proton never moves from one atom to another. By the end of this section, you will be able to: Remember that ions are formed only when. Does S Form A Positive Ion.
From www.biologyonline.com
Ion Definition and Examples Biology Online Dictionary Does S Form A Positive Ion By the end of this section, you will be able to: A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. Ionic compounds have positive ions and negative ions. Ionic formulas balance the total. Does S Form A Positive Ion.
From www.nagwa.com
Question Video Identifying What Changes When an Atom Forms a Positive Does S Form A Positive Ion Ions that contain fewer electrons than protons have a net positive charge and are called cations. Define ionic and molecular (covalent) compounds. A proton never moves from one atom to another. Ionic compounds have positive ions and negative ions. By the end of this section, you will be able to: Conversely, ions that contain more electrons than protons have a. Does S Form A Positive Ion.
From www.slideserve.com
PPT Crystal Binding (Bonding) Continued More on Ionic Bonding Does S Form A Positive Ion Remember that ions are formed only when electrons move from one atom to another; By the end of this section, you will be able to: Ionic formulas balance the total positive and negative charges. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms. Does S Form A Positive Ion.
From projectopenletter.com
Do Metals Form Positive Or Negative Ions Does S Form A Positive Ion Conversely, ions that contain more electrons than protons have a net negative charge and are. Define ionic and molecular (covalent) compounds. A proton never moves from one atom to another. Ionic compounds have positive ions and negative ions. By the end of this section, you will be able to: Ions form when atoms lose or gain electrons. A cation (a. Does S Form A Positive Ion.
From www.pinterest.com
Ion Names, Formulas and Charges Chart Teaching chemistry, Chemistry Does S Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A proton never moves from one atom to another. Ions that contain fewer electrons than protons have a net positive charge and are called cations. By the end of this section, you will. Does S Form A Positive Ion.
From ictchemskool.blogspot.com
SkooL of CheM Does S Form A Positive Ion Ionic formulas balance the total positive and negative charges. Ions form when atoms lose or gain electrons. By the end of this section, you will be able to: Remember that ions are formed only when electrons move from one atom to another; Define ionic and molecular (covalent) compounds. A cation (a positive ion) forms when a neutral atom loses one. Does S Form A Positive Ion.
From brokeasshome.com
Periodic Table Which Groups Of Elements Tend To Form Positive Ions Does S Form A Positive Ion By the end of this section, you will be able to: A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Ionic compounds have positive ions and negative ions. Ions form when atoms lose or gain electrons. Define ionic and molecular (covalent) compounds.. Does S Form A Positive Ion.
From gamma.app
Positive Ions in Chemistry and Its Characteristics Does S Form A Positive Ion By the end of this section, you will be able to: Conversely, ions that contain more electrons than protons have a net negative charge and are. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more. Does S Form A Positive Ion.
From www.youtube.com
Formation of positive ion YouTube Does S Form A Positive Ion Ions that contain fewer electrons than protons have a net positive charge and are called cations. Conversely, ions that contain more electrons than protons have a net negative charge and are. By the end of this section, you will be able to: A proton never moves from one atom to another. A cation (a positive ion) forms when a neutral. Does S Form A Positive Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does S Form A Positive Ion Ions form when atoms lose or gain electrons. Remember that ions are formed only when electrons move from one atom to another; A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A proton never moves from one atom to another. Ions that. Does S Form A Positive Ion.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Does S Form A Positive Ion Conversely, ions that contain more electrons than protons have a net negative charge and are. Define ionic and molecular (covalent) compounds. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. A proton never. Does S Form A Positive Ion.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Does S Form A Positive Ion By the end of this section, you will be able to: A proton never moves from one atom to another. Ions that contain fewer electrons than protons have a net positive charge and are called cations. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Does S Form A Positive Ion.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Does S Form A Positive Ion Ionic formulas balance the total positive and negative charges. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. Remember that ions are formed only when electrons move from one atom to another; A. Does S Form A Positive Ion.
From www.expii.com
Ions — Definition & Overview Expii Does S Form A Positive Ion Remember that ions are formed only when electrons move from one atom to another; Conversely, ions that contain more electrons than protons have a net negative charge and are. Ionic compounds have positive ions and negative ions. Ions that contain fewer electrons than protons have a net positive charge and are called cations. By the end of this section, you. Does S Form A Positive Ion.
From courses.lumenlearning.com
3.2 Ions The Basics of General, Organic, and Biological Chemistry Does S Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. By the end of this section, you will be able to: Remember that ions are formed only when electrons move from one atom to another; Ions form when atoms lose or gain electrons.. Does S Form A Positive Ion.
From www.nagwa.com
Question Video Comparing a Positive Ion to a Neutral Atom Nagwa Does S Form A Positive Ion Define ionic and molecular (covalent) compounds. Ionic compounds have positive ions and negative ions. Ionic formulas balance the total positive and negative charges. By the end of this section, you will be able to: A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms. Does S Form A Positive Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does S Form A Positive Ion Ionic compounds have positive ions and negative ions. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in. By the end of this section, you will be able to: A cation (a positive ion). Does S Form A Positive Ion.
From slideplayer.com
Ions, Valences and Oxidation Numbers ppt download Does S Form A Positive Ion Ions form when atoms lose or gain electrons. Define ionic and molecular (covalent) compounds. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Conversely, ions that. Does S Form A Positive Ion.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry Does S Form A Positive Ion Ionic compounds have positive ions and negative ions. A proton never moves from one atom to another. Conversely, ions that contain more electrons than protons have a net negative charge and are. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Remember that ions are formed only when electrons move from one atom. Does S Form A Positive Ion.