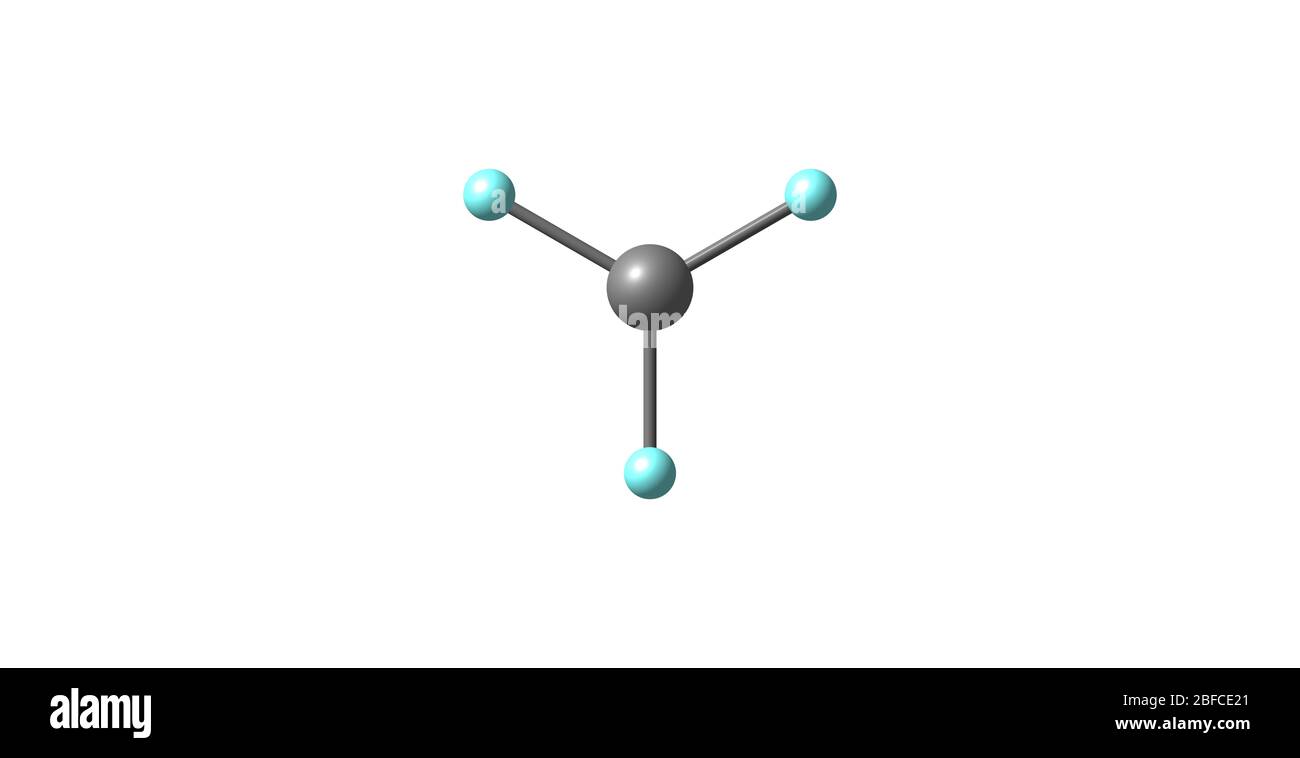
Oxygen Chlorine Trifluoride . It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. A highly reactive oxidant, it is. It reacts more violently than fluorine, often explosively. Essentially, in lamens terms, chlorine trifluoride can set fire. 12°c) which is irritating and toxic in the gaseous state. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3.
from www.alamy.com
Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. It reacts more violently than fluorine, often explosively. 12°c) which is irritating and toxic in the gaseous state. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. Essentially, in lamens terms, chlorine trifluoride can set fire. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. A highly reactive oxidant, it is.
Chlorine trifluoride is an interhalogen compound with the formula ClF3
Oxygen Chlorine Trifluoride 12°c) which is irritating and toxic in the gaseous state. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. It reacts more violently than fluorine, often explosively. It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. A highly reactive oxidant, it is. Essentially, in lamens terms, chlorine trifluoride can set fire. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. 12°c) which is irritating and toxic in the gaseous state.
From www.youtube.com
ClF3 Hybridization (Chlorine Trifluoride) YouTube Oxygen Chlorine Trifluoride Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. It reacts more violently than fluorine, often explosively. 12°c) which is irritating and toxic in the gaseous state. Essentially, in lamens terms, chlorine trifluoride can set fire. A highly reactive oxidant, it is. As it turns out, the chemical is more oxidizing than. Oxygen Chlorine Trifluoride.
From www.nagwa.com
Question Video Understanding How Ammonia Molecules Bond Boron Oxygen Chlorine Trifluoride It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. 12°c) which is irritating and toxic in the gaseous state. It reacts more violently than fluorine, often explosively. Chlorine oxide trifluoride or chlorine trifluoride. Oxygen Chlorine Trifluoride.
From favpng.com
Vanadium Oxytrifluoride Oxygen Difluoride Oxygen Fluoride, PNG Oxygen Chlorine Trifluoride Essentially, in lamens terms, chlorine trifluoride can set fire. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. It reacts more violently than fluorine, often explosively. It is made by reacting. Oxygen Chlorine Trifluoride.
From www.youtube.com
ClF3 (Chlorine trifluoride) Molecular Geometry, Bond Angles,& Electron Oxygen Chlorine Trifluoride To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. 12°c) which is irritating and toxic in the gaseous state. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. A highly reactive oxidant, it is. Chlorine oxide trifluoride or chlorine trifluoride. Oxygen Chlorine Trifluoride.
From www.studocu.com
Chemistry 101 Chapter 7 Part 3 Draw the Lewis structure of ClF₃ Oxygen Chlorine Trifluoride Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. Essentially, in lamens terms, chlorine trifluoride can set fire. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. 12°c) which is irritating and toxic in the gaseous state. Chlorine oxide trifluoride. Oxygen Chlorine Trifluoride.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Oxygen Chlorine Trifluoride To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. A highly reactive oxidant, it is. It is made by reacting chlorine with an excess of fluorine at 250° c. Oxygen Chlorine Trifluoride.
From www.researchgate.net
Reactor and samples in this study. (a) Gases and reactor for exposing Oxygen Chlorine Trifluoride Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. Essentially, in lamens terms, chlorine trifluoride can set fire. A highly reactive oxidant, it is. 12°c) which is irritating and toxic in the. Oxygen Chlorine Trifluoride.
From www.researchgate.net
Sample appearance along with exposure to chlorine trifluoride gas and Oxygen Chlorine Trifluoride As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. A highly reactive oxidant,. Oxygen Chlorine Trifluoride.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Oxygen Chlorine Trifluoride Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. It reacts more violently than fluorine, often explosively. 12°c) which is irritating and toxic in the gaseous state. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. Essentially, in. Oxygen Chlorine Trifluoride.
From www.dreamstime.com
Chlorine Trifluoride Dangerous Poisonous Gas in Chemical Glassware Oxygen Chlorine Trifluoride A highly reactive oxidant, it is. Essentially, in lamens terms, chlorine trifluoride can set fire. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. 12°c) which is irritating and toxic in. Oxygen Chlorine Trifluoride.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Oxygen Chlorine Trifluoride Essentially, in lamens terms, chlorine trifluoride can set fire. It reacts more violently than fluorine, often explosively. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. It is made by reacting chlorine with an. Oxygen Chlorine Trifluoride.
From www.researchgate.net
Appearance and contents of silicon, carbon, oxygen, nitrogen, fluorine Oxygen Chlorine Trifluoride It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. It reacts more violently than fluorine, often explosively. 12°c) which is irritating and toxic in the gaseous state. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. As it turns out, the chemical is. Oxygen Chlorine Trifluoride.
From www.studocu.com
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu Oxygen Chlorine Trifluoride To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. 12°c) which is irritating and toxic in the gaseous state. It reacts more violently than fluorine, often explosively. A highly reactive oxidant, it is. Essentially, in lamens terms, chlorine trifluoride can set fire. As it turns out, the chemical is. Oxygen Chlorine Trifluoride.
From www.kindpng.com
Chlorine Trifluoride 3d Balls Chlorine Trifluoride 3d Structure, HD Oxygen Chlorine Trifluoride Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. It reacts more violently than fluorine, often explosively. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. 12°c) which is irritating and toxic in the gaseous state. A highly. Oxygen Chlorine Trifluoride.
From bilag.xxl.no
Draw The Lewis Structure For The Chlorine Trifluoride Molecule Oxygen Chlorine Trifluoride A highly reactive oxidant, it is. It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind. Oxygen Chlorine Trifluoride.
From chemicalportal.ru
Фторид хлора (III) — свойства, получение и применение Oxygen Chlorine Trifluoride It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. 12°c) which is irritating and toxic in the gaseous state. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and. Oxygen Chlorine Trifluoride.
From interestingengineering.com
Chlorine Trifluoride The Compound That Can Even Set Fire to Glass Oxygen Chlorine Trifluoride Essentially, in lamens terms, chlorine trifluoride can set fire. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. A highly reactive oxidant, it is. It reacts more violently than fluorine, often explosively. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid.. Oxygen Chlorine Trifluoride.
From www.procurementresource.com
Chlorine Trifluoride Production Cost Analysis Reports 2024 Oxygen Chlorine Trifluoride Essentially, in lamens terms, chlorine trifluoride can set fire. A highly reactive oxidant, it is. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. It reacts more violently than. Oxygen Chlorine Trifluoride.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Oxygen Chlorine Trifluoride Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. It reacts. Oxygen Chlorine Trifluoride.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Oxygen Chlorine Trifluoride Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. A highly. Oxygen Chlorine Trifluoride.
From www.wikiwand.com
Chlorine trifluoride oxide Wikiwand Oxygen Chlorine Trifluoride Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. As it turns. Oxygen Chlorine Trifluoride.
From www.alamy.com
Chlorine gas white background hires stock photography and images Alamy Oxygen Chlorine Trifluoride As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. 12°c) which is irritating and toxic in the gaseous state. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. To understand why chlorine trifluoride is top of the avoid at all costs list, let's. Oxygen Chlorine Trifluoride.
From www.anyrgb.com
Adduct, phosphoryl Chloride, boron Trifluoride, lewis Acids And Bases Oxygen Chlorine Trifluoride As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. A highly reactive oxidant, it is. It reacts more violently than fluorine, often explosively. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. Essentially, in lamens terms, chlorine trifluoride can set. Oxygen Chlorine Trifluoride.
From www.researchgate.net
(PDF) Lowest Concentration of Chlorine Trifluoride Gas for Cleaning Oxygen Chlorine Trifluoride To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. A highly reactive oxidant, it is. Essentially, in lamens terms, chlorine trifluoride can set fire. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. Chlorine oxide trifluoride or chlorine. Oxygen Chlorine Trifluoride.
From www.youtube.com
ClF3 Lewis Structure How to Draw the Lewis Structure for ClF3 YouTube Oxygen Chlorine Trifluoride 12°c) which is irritating and toxic in the gaseous state. It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. A highly reactive oxidant, it is. Essentially, in lamens terms, chlorine trifluoride. Oxygen Chlorine Trifluoride.
From de-academic.com
Chlor(III)fluorid Oxygen Chlorine Trifluoride To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. 12°c) which is irritating and toxic in the gaseous state. It reacts more violently than fluorine, often explosively. It is made by reacting. Oxygen Chlorine Trifluoride.
From favpng.com
Chlorine Trifluoride Dichlorine Monoxide Chemistry Chloride, PNG Oxygen Chlorine Trifluoride Essentially, in lamens terms, chlorine trifluoride can set fire. It reacts more violently than fluorine, often explosively. A highly reactive oxidant, it is. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent.. Oxygen Chlorine Trifluoride.
From www.researchgate.net
12. Fraction of carbon, silicon, oxygen, chlorine and fluorine on the Oxygen Chlorine Trifluoride Essentially, in lamens terms, chlorine trifluoride can set fire. 12°c) which is irritating and toxic in the gaseous state. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. It is made by reacting chlorine. Oxygen Chlorine Trifluoride.
From www.alamy.com
Chlorine trifluoride hires stock photography and images Alamy Oxygen Chlorine Trifluoride A highly reactive oxidant, it is. It reacts more violently than fluorine, often explosively. 12°c) which is irritating and toxic in the gaseous state. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind. Oxygen Chlorine Trifluoride.
From dokumen.tips
(PDF) Carbon dioxidewater oxygen isotope fractionation factor using Oxygen Chlorine Trifluoride Essentially, in lamens terms, chlorine trifluoride can set fire. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. 12°c) which is irritating and toxic in the gaseous state. It is made by reacting chlorine with an excess of fluorine at 250° c in a nickel tube. Chlorine oxide trifluoride. Oxygen Chlorine Trifluoride.
From www.dreamstime.com
Chlorine Trifluoride Gas Molecule Icon Stock Illustration Oxygen Chlorine Trifluoride Essentially, in lamens terms, chlorine trifluoride can set fire. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. A highly reactive oxidant, it is. To understand why chlorine trifluoride is top of. Oxygen Chlorine Trifluoride.
From www.youtube.com
What is chlorine trifluoride in hindi। how to make chlorine trifluoride Oxygen Chlorine Trifluoride A highly reactive oxidant, it is. 12°c) which is irritating and toxic in the gaseous state. Essentially, in lamens terms, chlorine trifluoride can set fire. It reacts more violently than fluorine, often explosively. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. To understand why chlorine trifluoride is top of the avoid. Oxygen Chlorine Trifluoride.
From www.shutterstock.com
Chlorine Trifluoride Molecular Structure Formula Periodic Stock Vector Oxygen Chlorine Trifluoride It reacts more violently than fluorine, often explosively. 12°c) which is irritating and toxic in the gaseous state. As it turns out, the chemical is more oxidizing than oxygen itself, making it an extremely effective explosive. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. Chlorine oxide trifluoride or. Oxygen Chlorine Trifluoride.
From www.researchgate.net
Surface appearances changed by the chlorine trifluoride etching at Oxygen Chlorine Trifluoride It reacts more violently than fluorine, often explosively. To understand why chlorine trifluoride is top of the avoid at all costs list, let's rewind to the oxidizing agent. Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular. Oxygen Chlorine Trifluoride.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Oxygen Chlorine Trifluoride Chlorine trifluoride (clf 3) is a colorless gas that condenses to a green liquid and freezes to a white solid. Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula clof 3. Essentially, in lamens terms, chlorine trifluoride can set fire. It is made by reacting chlorine with an excess of fluorine at 250° c. Oxygen Chlorine Trifluoride.