Temperature Of Boiling Water And Steam . Other definitions say that steam is water vapor if the. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. But, the boiling point of water changes with. The “normal” refers to sea level or an elevation of 0 meters or feet. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. In fact, at the microscopic level, there may be cooler regions of boiling water. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k.
from www.chegg.com
The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. But, the boiling point of water changes with. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The “normal” refers to sea level or an elevation of 0 meters or feet. In fact, at the microscopic level, there may be cooler regions of boiling water. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. Other definitions say that steam is water vapor if the. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which.
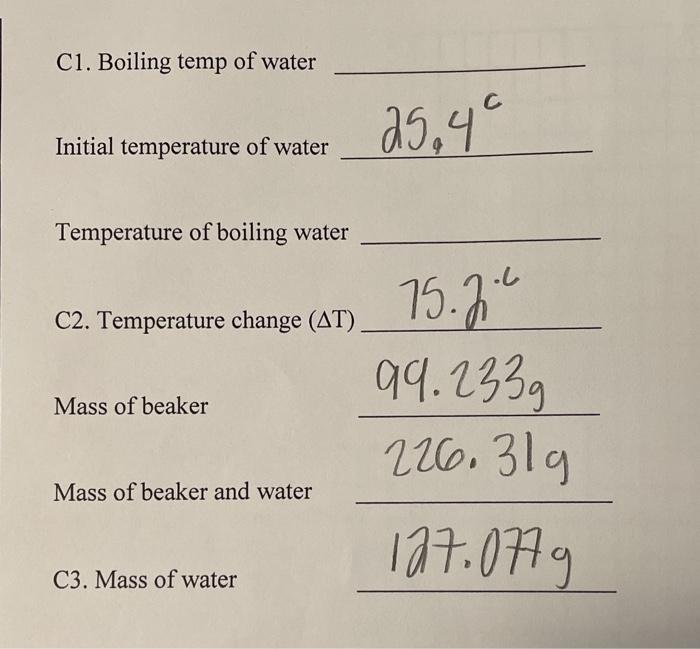
Solved C1. Boiling temp of water 25,49 Initial temperature
Temperature Of Boiling Water And Steam The “normal” refers to sea level or an elevation of 0 meters or feet. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. But, the boiling point of water changes with. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. In fact, at the microscopic level, there may be cooler regions of boiling water. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The “normal” refers to sea level or an elevation of 0 meters or feet. Other definitions say that steam is water vapor if the. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing.
From recipepes.com
what temperature does water boil Temperature Of Boiling Water And Steam In fact, at the microscopic level, there may be cooler regions of boiling water. But, the boiling point of water changes with. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. The “normal” refers to sea level or an elevation of. Temperature Of Boiling Water And Steam.
From sciencenotes.org
How to Boil Water at Room Temperature Temperature Of Boiling Water And Steam When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The 'boiling. Temperature Of Boiling Water And Steam.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Temperature Of Boiling Water And Steam But, the boiling point of water changes with. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Other definitions say that steam is water vapor if the. The 'boiling point' of water is the temperature at which steam. Temperature Of Boiling Water And Steam.
From socratic.org
What is the profile of the graph of temperature versus time, when water Temperature Of Boiling Water And Steam When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. But, the boiling point of water changes with. In fact, at the microscopic level, there may be cooler regions of boiling water. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the. Temperature Of Boiling Water And Steam.
From www.youtube.com
Energy required reach the boiling temperature of water or to reach 100 Temperature Of Boiling Water And Steam But, the boiling point of water changes with. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. In. Temperature Of Boiling Water And Steam.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Temperature Of Boiling Water And Steam The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. The “normal” refers to sea level or an elevation of 0 meters or feet. When vapor bubbles form near a heat source, like at the bottom. Temperature Of Boiling Water And Steam.
From www.chegg.com
Solved C1. Boiling temp of water 25,49 Initial temperature Temperature Of Boiling Water And Steam When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. But, the boiling point of water changes with. The 'boiling point' of water is the temperature at which steam and liquid exist at. Temperature Of Boiling Water And Steam.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free Temperature Of Boiling Water And Steam But, the boiling point of water changes with. Other definitions say that steam is water vapor if the. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The 'boiling point' of. Temperature Of Boiling Water And Steam.
From www.pinterest.com
Mastering Boiling Water Tips for Cooking at Altitude Temperature Of Boiling Water And Steam The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. Other definitions say that steam is water vapor if the. When water is heated at atmospheric pressure, its temperature rises until it. Temperature Of Boiling Water And Steam.
From www.youtube.com
At What Temperature Does Water Steam? Know Now YouTube Temperature Of Boiling Water And Steam The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these. Temperature Of Boiling Water And Steam.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Temperature Of Boiling Water And Steam The “normal” refers to sea level or an elevation of 0 meters or feet. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the. Temperature Of Boiling Water And Steam.
From hikingmastery.com
Does Boiling Water Purify It Basic Facts and Useful Temperature Of Boiling Water And Steam But, the boiling point of water changes with. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The normal boiling point of. Temperature Of Boiling Water And Steam.
From sciencenotes.org
How to Boil Water at Room Temperature Temperature Of Boiling Water And Steam The “normal” refers to sea level or an elevation of 0 meters or feet. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. But, the boiling point of water changes with. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. In. Temperature Of Boiling Water And Steam.
From howchimp.com
What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit Temperature Of Boiling Water And Steam The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. In fact, at the microscopic level, there may be cooler regions of boiling water. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Other. Temperature Of Boiling Water And Steam.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Temperature Of Boiling Water And Steam If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. The “normal” refers to sea level or an elevation of 0 meters or feet. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the. Temperature Of Boiling Water And Steam.
From www.yaclass.in
Activity on temperature — lesson. Science CBSE, Class 7. Temperature Of Boiling Water And Steam Other definitions say that steam is water vapor if the. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. The “normal” refers to sea. Temperature Of Boiling Water And Steam.
From www.foodabovegold.com
Perfectly Cooked by Steaming Cooking Methods 101 Food Above Gold Temperature Of Boiling Water And Steam When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. In fact, at the microscopic level, there may be cooler regions of boiling water. But, the boiling point of water changes with. The water may boil more vigorously and convert into steam more quickly, but it. Temperature Of Boiling Water And Steam.
From recipepes.com
steam temperature chart Temperature Of Boiling Water And Steam But, the boiling point of water changes with. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. The “normal” refers to sea level or an elevation of 0 meters or feet. In fact, at the microscopic level, there may be cooler regions of boiling water. The water may boil. Temperature Of Boiling Water And Steam.
From studylib.net
Lab Boiling Temperature of Water Temperature Of Boiling Water And Steam But, the boiling point of water changes with. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Other definitions say that steam is water vapor if the. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. In fact, at the microscopic level, there may. Temperature Of Boiling Water And Steam.
From www.animalia-life.club
Boiling Point Of Water For Kids Temperature Of Boiling Water And Steam If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. Other definitions say that steam is water vapor if the. In fact, at the microscopic level, there may be cooler. Temperature Of Boiling Water And Steam.
From www.gkseries.com
Temperature of boiling water is measured by a ____ thermometer Temperature Of Boiling Water And Steam When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. But, the boiling point of water changes with. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these. Temperature Of Boiling Water And Steam.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Temperature Of Boiling Water And Steam Other definitions say that steam is water vapor if the. In fact, at the microscopic level, there may be cooler regions of boiling water. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. When vapor. Temperature Of Boiling Water And Steam.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts Temperature Of Boiling Water And Steam The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. In fact, at the microscopic level, there may be cooler regions of boiling water. The 'boiling point' of water is the temperature at which steam and liquid exist at. Temperature Of Boiling Water And Steam.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG Temperature Of Boiling Water And Steam But, the boiling point of water changes with. The “normal” refers to sea level or an elevation of 0 meters or feet. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. The. Temperature Of Boiling Water And Steam.
From blog.sciencescore.com
What temperature does water boil? Temperature Of Boiling Water And Steam When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Other definitions say that steam is water vapor if the. The water. Temperature Of Boiling Water And Steam.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent Temperature Of Boiling Water And Steam The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. In fact, at the microscopic level, there may be cooler regions of boiling water. The water may boil. Temperature Of Boiling Water And Steam.
From www.teachoo.com
What produces more severe burns, boiling water or steam? (Teachoo) Temperature Of Boiling Water And Steam If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. The 'boiling point' of water is the temperature at which steam and liquid. Temperature Of Boiling Water And Steam.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning Temperature Of Boiling Water And Steam The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Other definitions say that steam is water vapor if the. But, the boiling point of water changes with. The “normal” refers to sea level or an elevation of 0 meters or feet.. Temperature Of Boiling Water And Steam.
From www.vernier.com
Boiling Temperature of Water > Experiment 3 from Exploring Physical Science Temperature Of Boiling Water And Steam The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. In fact, at the microscopic level, there may be cooler regions of boiling water. When vapor bubbles form near a heat source, like at the bottom of a pot,. Temperature Of Boiling Water And Steam.
From www.boiler-planning.com
Presión y temperatura Bosch Planificación de calderas de vapor Temperature Of Boiling Water And Steam If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. But, the boiling point of water changes with. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. The. Temperature Of Boiling Water And Steam.
From www.boiler-planning.com
Steam types Bosch steam boiler planning Commercial & Industrial Temperature Of Boiling Water And Steam In fact, at the microscopic level, there may be cooler regions of boiling water. If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the. Temperature Of Boiling Water And Steam.
From www.researchgate.net
Densities of saturated water and steam vapour in the vicinity of the Temperature Of Boiling Water And Steam The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. When vapor bubbles form near a heat source, like at the bottom of a pot, the gas bubbles insulate the water from the heat. But, the. Temperature Of Boiling Water And Steam.
From mavink.com
Water Boiling Pressure Chart Temperature Of Boiling Water And Steam But, the boiling point of water changes with. The “normal” refers to sea level or an elevation of 0 meters or feet. Other definitions say that steam is water vapor if the. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. When vapor bubbles form near a heat source, like at the bottom of a. Temperature Of Boiling Water And Steam.
From www.researchgate.net
3 Changes in temperature of the boiling point of water with deepness Temperature Of Boiling Water And Steam The water may boil more vigorously and convert into steam more quickly, but it won’t get hotter. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Other definitions say that steam is water vapor if the. In fact, at the microscopic level, there may be cooler regions of boiling water. But, the boiling point of. Temperature Of Boiling Water And Steam.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Temperature Of Boiling Water And Steam If you boil water, you do indeed release packets of 100°c water vapour into the air, but these immediately cool down when mixing. When water is heated at atmospheric pressure, its temperature rises until it reaches 212°f (100°c), the highest temperature at which. Other definitions say that steam is water vapor if the. But, the boiling point of water changes. Temperature Of Boiling Water And Steam.