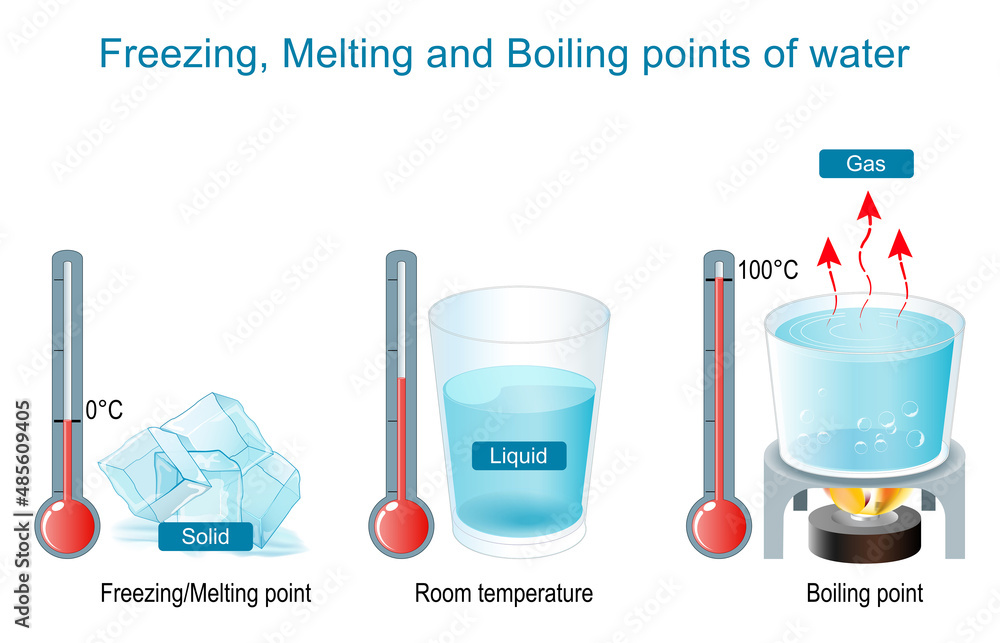
What Causes The Boiling And Freezing Point Of Water To Be So High . Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. Here, δt b represents the elevation in the boiling point of the solution. Indicate what happens to the boiling. Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Explain what the term colligative means, and list the colligative properties. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The depression of the freezing point of water by a. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water.
from stock.adobe.com
Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. The depression of the freezing point of water by a. Explain what the term colligative means, and list the colligative properties. Here, δt b represents the elevation in the boiling point of the solution. Indicate what happens to the boiling. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water.
Boiling and Evaporation, Freezing and Melting Points of Water. Stock
What Causes The Boiling And Freezing Point Of Water To Be So High Indicate what happens to the boiling. Indicate what happens to the boiling. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The depression of the freezing point of water by a. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. Explain what the term colligative means, and list the colligative properties. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Here, δt b represents the elevation in the boiling point of the solution.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What Causes The Boiling And Freezing Point Of Water To Be So High Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Indicate what happens to the boiling. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. Learn how the boiling point of a solution. What Causes The Boiling And Freezing Point Of Water To Be So High.
From exoudndgb.blob.core.windows.net
What Is The Freezing Point Of Whiskey at Katie Wilson blog What Causes The Boiling And Freezing Point Of Water To Be So High Explain what the term colligative means, and list the colligative properties. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. Indicate. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Causes The Boiling And Freezing Point Of Water To Be So High Explain what the term colligative means, and list the colligative properties. Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles.. What Causes The Boiling And Freezing Point Of Water To Be So High.
From general.chemistrysteps.com
Colligative Properties Practice Problems Chemistry Steps What Causes The Boiling And Freezing Point Of Water To Be So High Explain what the term colligative means, and list the colligative properties. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena.. What Causes The Boiling And Freezing Point Of Water To Be So High.
From ceayhytg.blob.core.windows.net
Gas Vs Electric Boiling Water at Fredric Williams blog What Causes The Boiling And Freezing Point Of Water To Be So High The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. Here, δt b represents the elevation in the boiling point of the solution. Explain what the term colligative means, and list the colligative properties. Learn how the boiling point of a solution is higher. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.pinterest.co.uk
Image result for boiling freezing melting anchor chart Teaching What Causes The Boiling And Freezing Point Of Water To Be So High The depression of the freezing point of water by a. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Indicate what happens to the boiling. Learn how solutes in water lower the freezing point and raise the boiling. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.wave3.com
Behind the Forecast Outwitting the weather when baking What Causes The Boiling And Freezing Point Of Water To Be So High Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Here, δt b represents the elevation in the boiling point of the solution. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile. What Causes The Boiling And Freezing Point Of Water To Be So High.
From inspiritvr.com
Boiling Point Elevation and Freezing Point Depression Study Guide What Causes The Boiling And Freezing Point Of Water To Be So High The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Here, δt b represents the elevation in the boiling point of the solution. Explain what the term colligative means, and list the colligative properties. The web page explains why. What Causes The Boiling And Freezing Point Of Water To Be So High.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Causes The Boiling And Freezing Point Of Water To Be So High The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. Here, δt b represents the elevation in the boiling point of the. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Causes The Boiling And Freezing Point Of Water To Be So High Indicate what happens to the boiling. The depression of the freezing point of water by a. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. Explain what the term colligative means, and list the colligative properties. Here, δt b represents. What Causes The Boiling And Freezing Point Of Water To Be So High.
From scienceblogs.com
Water in Space What Happens? ScienceBlogs What Causes The Boiling And Freezing Point Of Water To Be So High Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Explain what the term colligative means, and list the colligative properties. Here, δt b represents the elevation in the boiling point of the solution. Indicate what happens to the boiling. The web page explains why water has a much higher. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer What Causes The Boiling And Freezing Point Of Water To Be So High Here, δt b represents the elevation in the boiling point of the solution. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.liveworksheets.com
MELTING and FREEZING 2163323 lailani gamban Live What Causes The Boiling And Freezing Point Of Water To Be So High Indicate what happens to the boiling. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. Here, δt b. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.gettyimages.ae
Freezing And Boiling Points In Celsius And Fahrenheit HighRes Vector What Causes The Boiling And Freezing Point Of Water To Be So High The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Learn how the boiling point of a. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.youtube.com
Freezing and Boiling Point Graph YouTube What Causes The Boiling And Freezing Point Of Water To Be So High The depression of the freezing point of water by a. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. Here, δt. What Causes The Boiling And Freezing Point Of Water To Be So High.
From hxehubabb.blob.core.windows.net
What Is The Temperature In Degrees Fahrenheit Of A Freezer Kept At 20 C What Causes The Boiling And Freezing Point Of Water To Be So High Explain what the term colligative means, and list the colligative properties. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure. What Causes The Boiling And Freezing Point Of Water To Be So High.
From circuitwiringstroke123.z13.web.core.windows.net
Normal Boiling Point Chemistry What Causes The Boiling And Freezing Point Of Water To Be So High Explain what the term colligative means, and list the colligative properties. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. The depression of the freezing point of water by a. Learn how solutes in water lower the freezing point and raise the boiling. What Causes The Boiling And Freezing Point Of Water To Be So High.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Causes The Boiling And Freezing Point Of Water To Be So High The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. Learn how solutes in water lower the. What Causes The Boiling And Freezing Point Of Water To Be So High.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Causes The Boiling And Freezing Point Of Water To Be So High Explain what the term colligative means, and list the colligative properties. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Learn how the boiling point of a solution is higher than that of the pure solvent due to. What Causes The Boiling And Freezing Point Of Water To Be So High.
From pressbooks.online.ucf.edu
12.5 Colligative Properties Chemistry Fundamentals What Causes The Boiling And Freezing Point Of Water To Be So High Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. Explain what the term colligative means, and list the colligative properties. Indicate what happens to the boiling. The. What Causes The Boiling And Freezing Point Of Water To Be So High.
From theconversation.com
Salt doesn't melt ice here's how it actually makes winter streets safe What Causes The Boiling And Freezing Point Of Water To Be So High Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Here, δt b represents the elevation in the boiling point of the solution. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. The. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.youtube.com
Why is the Boiling Point of water (H2O) so high? YouTube What Causes The Boiling And Freezing Point Of Water To Be So High Indicate what happens to the boiling. Explain what the term colligative means, and list the colligative properties. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. Here, δt b represents the elevation in the boiling point of the solution. The. What Causes The Boiling And Freezing Point Of Water To Be So High.
From sciencenotes.org
What Is the Freezing Point of Water? Fahrenheit, Celsius, and Kelvin What Causes The Boiling And Freezing Point Of Water To Be So High Explain what the term colligative means, and list the colligative properties. Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. Indicate what happens to the boiling. Here,. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Causes The Boiling And Freezing Point Of Water To Be So High The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Explain what the term colligative means, and list the. What Causes The Boiling And Freezing Point Of Water To Be So High.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock What Causes The Boiling And Freezing Point Of Water To Be So High The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. Learn how solutes in water lower the freezing point and raise the. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Causes The Boiling And Freezing Point Of Water To Be So High The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Indicate what happens to the boiling. Here, δt b represents the elevation in the boiling point of the solution. Learn how solutes in water lower the freezing point and. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.animalia-life.club
Boiling Point Of Water For Kids What Causes The Boiling And Freezing Point Of Water To Be So High The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. The web page explains why water has a much higher boiling point than expected based on its size, using a diagram and. Indicate what happens to the boiling. The depression of the freezing point. What Causes The Boiling And Freezing Point Of Water To Be So High.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Causes The Boiling And Freezing Point Of Water To Be So High Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. The particles of solute block the crystallization of water, at least. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Causes The Boiling And Freezing Point Of Water To Be So High Explain what the term colligative means, and list the colligative properties. Here, δt b represents the elevation in the boiling point of the solution. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Indicate what happens to the. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.pinterest.com
Boiling Point/Freezing/ Melting Point AnchorChart Fifth Grade Science What Causes The Boiling And Freezing Point Of Water To Be So High Indicate what happens to the boiling. Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Learn how the boiling point of a solution is higher than that of the pure solvent due to the decrease in vapor pressure caused by the nonvolatile solute particles. The web page explains why. What Causes The Boiling And Freezing Point Of Water To Be So High.
From www.thoughtco.com
What Is the Freezing Point of Water? What Causes The Boiling And Freezing Point Of Water To Be So High The particles of solute block the crystallization of water, at least up until a certain point, causing a depression in the freezing point of the water. The depression of the freezing point of water by a. Learn how solutes in water lower the freezing point and raise the boiling point, and how pressure affects these phenomena. Indicate what happens to. What Causes The Boiling And Freezing Point Of Water To Be So High.