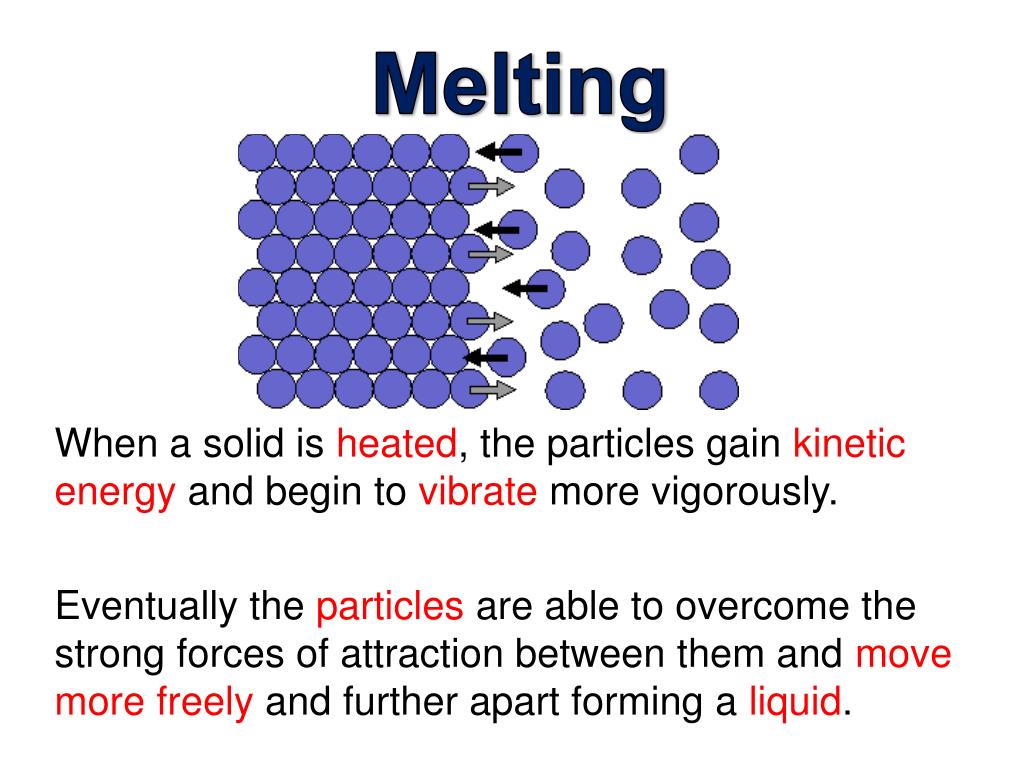
What Happens When Ice Melts In Terms Of Energy . The ice cubes are at the melting temperature of 0 °c °c. Heat is transferred from the soda to the ice for melting. For example, liquid water turns into steam when it is heated enough, and. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. Substances can change state, usually when they are heated or cooled. Melting of ice occurs in two steps:. At the melting point, they have enough kinetic. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7.
from www.slideserve.com
Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. Substances can change state, usually when they are heated or cooled. Heat is transferred from the soda to the ice for melting. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. For example, liquid water turns into steam when it is heated enough, and. The ice cubes are at the melting temperature of 0 °c °c. Melting of ice occurs in two steps:. At the melting point, they have enough kinetic.
PPT The Nature of Matter PowerPoint Presentation, free download ID
What Happens When Ice Melts In Terms Of Energy For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. Heat is transferred from the soda to the ice for melting. Melting of ice occurs in two steps:. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. The ice cubes are at the melting temperature of 0 °c °c. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. Substances can change state, usually when they are heated or cooled. At the melting point, they have enough kinetic. For example, liquid water turns into steam when it is heated enough, and. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7.
From ar.inspiredpencil.com
Melting Point Of Ice What Happens When Ice Melts In Terms Of Energy Heat is transferred from the soda to the ice for melting. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. Substances can change state, usually when they are heated or cooled. For example, liquid water turns into steam when it is heated enough, and. The ice cubes are at the melting temperature of 0 °c °c.. What Happens When Ice Melts In Terms Of Energy.
From www.youtube.com
What Happens When Ice Melts In Different Liquids? YouTube What Happens When Ice Melts In Terms Of Energy Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. Heat is transferred from the soda to the ice for melting. For example, liquid water turns into. What Happens When Ice Melts In Terms Of Energy.
From socratic.org
What happens to the energy of its molecules as ice melts into What Happens When Ice Melts In Terms Of Energy As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. Melting of ice occurs in two steps:. At the melting point, they have enough kinetic. For solid water (ice), the melting point is 0. What Happens When Ice Melts In Terms Of Energy.
From www.numerade.com
SOLVED Which statement accurately describes what happens when ice What Happens When Ice Melts In Terms Of Energy 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. Substances can change state, usually when they. What Happens When Ice Melts In Terms Of Energy.
From www.youtube.com
What happens to water level, when ice melts। NEET/JEE। Physics।Fluid What Happens When Ice Melts In Terms Of Energy For example, liquid water turns into steam when it is heated enough, and. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. At the melting point, they have enough kinetic. Heat is transferred from the soda to the ice for melting. The ice. What Happens When Ice Melts In Terms Of Energy.
From gioanhvbu.blob.core.windows.net
What Happens To Volume Of Water When Ice Melts at John Girard blog What Happens When Ice Melts In Terms Of Energy As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. Melting of ice occurs in two steps:. Substances can change state, usually when they are heated or cooled. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. 1, we. What Happens When Ice Melts In Terms Of Energy.
From www.vecteezy.com
Diagram showing how ice melts 433823 Vector Art at Vecteezy What Happens When Ice Melts In Terms Of Energy The ice cubes are at the melting temperature of 0 °c °c. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. Substances can change state, usually. What Happens When Ice Melts In Terms Of Energy.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) What Happens When Ice Melts In Terms Of Energy Melting of ice occurs in two steps:. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. Substances can change state, usually when they are heated or cooled. At the melting point, they have enough kinetic. For example, liquid water turns into steam when it is heated enough, and. 1, we find that. What Happens When Ice Melts In Terms Of Energy.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID What Happens When Ice Melts In Terms Of Energy For example, liquid water turns into steam when it is heated enough, and. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. The ice cubes are at the melting temperature of 0 °c °c. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them. What Happens When Ice Melts In Terms Of Energy.
From studylib.net
What Happens to Molecules When a Substance Melts? Ice Liquid What Happens When Ice Melts In Terms Of Energy Substances can change state, usually when they are heated or cooled. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. Melting of ice occurs in two steps:. Heat is transferred from the soda to the ice for melting. For solid water (ice), the melting point is 0 °c., the energy gained by. What Happens When Ice Melts In Terms Of Energy.
From brainly.com
PLEASE HELP ASAPP Which statement accurately describes what happens What Happens When Ice Melts In Terms Of Energy At the melting point, they have enough kinetic. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. Substances can change state, usually when they are heated. What Happens When Ice Melts In Terms Of Energy.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition What Happens When Ice Melts In Terms Of Energy Heat is transferred from the soda to the ice for melting. Substances can change state, usually when they are heated or cooled. At the melting point, they have enough kinetic. Melting of ice occurs in two steps:. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong. What Happens When Ice Melts In Terms Of Energy.
From www.shmoop.com
Chemistry Phase Changes Shmoop Chemistry What Happens When Ice Melts In Terms Of Energy 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. For example, liquid water turns into steam when it is heated enough, and. Heat is transferred from the soda to the ice for melting. Substances can change state, usually when they are heated or cooled. The ice cubes are at the melting temperature of 0 °c °c.. What Happens When Ice Melts In Terms Of Energy.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens When Ice Melts In Terms Of Energy 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. The ice cubes are at the melting temperature of 0 °c °c. For example, liquid water turns into steam when it is heated enough, and. Heat is transferred from the soda to the ice for melting. Using the equation for a change in temperature and the value. What Happens When Ice Melts In Terms Of Energy.
From sealevel.nasa.gov
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass What Happens When Ice Melts In Terms Of Energy Substances can change state, usually when they are heated or cooled. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. For example, liquid water turns into steam when it is heated enough, and.. What Happens When Ice Melts In Terms Of Energy.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science What Happens When Ice Melts In Terms Of Energy Melting of ice occurs in two steps:. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. At the melting point, they have enough kinetic. Substances can change state, usually when they are heated or cooled. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. For solid water (ice),. What Happens When Ice Melts In Terms Of Energy.
From www.slideshare.net
Heat What Happens When Ice Melts In Terms Of Energy For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. Melting of ice occurs in two steps:. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. For example, liquid water turns into steam when it is heated enough, and. Using. What Happens When Ice Melts In Terms Of Energy.
From www.slideserve.com
PPT Energy and Chemical Change PowerPoint Presentation, free download What Happens When Ice Melts In Terms Of Energy 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. For example, liquid water turns into steam when it is heated enough, and. Substances can change state, usually when they are heated or cooled. Heat is transferred from the soda to the ice for melting. The ice cubes are at the melting temperature of 0 °c °c.. What Happens When Ice Melts In Terms Of Energy.
From studybrewmaster.z21.web.core.windows.net
What Happens To A Solid When It Melts What Happens When Ice Melts In Terms Of Energy For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. Heat is transferred from the soda to the ice for melting. The ice cubes are at the melting temperature of 0 °c °c. At the melting point, they have enough kinetic. 1, we find. What Happens When Ice Melts In Terms Of Energy.
From gioanhvbu.blob.core.windows.net
What Happens To Volume Of Water When Ice Melts at John Girard blog What Happens When Ice Melts In Terms Of Energy Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. For example, liquid water turns into steam when it is heated enough, and. Substances can change state, usually when they. What Happens When Ice Melts In Terms Of Energy.
From news.schoolsdo.org
When ice melts on Earth, it happens layer by layer Voxitatis Blog What Happens When Ice Melts In Terms Of Energy Substances can change state, usually when they are heated or cooled. Melting of ice occurs in two steps:. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. For solid water (ice), the melting point is 0 °c., the energy. What Happens When Ice Melts In Terms Of Energy.
From ian.umces.edu
Experiment Demonstrating Melting Ice Media Library Integration and What Happens When Ice Melts In Terms Of Energy Melting of ice occurs in two steps:. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. For example, liquid water turns into steam when it is heated enough, and. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. As the temperature of the ice increases, the water molecules. What Happens When Ice Melts In Terms Of Energy.
From stock.adobe.com
Thermal energy physics definition, example with water and What Happens When Ice Melts In Terms Of Energy 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. Substances can change state, usually when they are heated or cooled. For example, liquid water turns into steam when it is heated enough, and. Heat is transferred from the soda to the ice for melting. As the temperature of the ice increases, the water molecules in the. What Happens When Ice Melts In Terms Of Energy.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) What Happens When Ice Melts In Terms Of Energy The ice cubes are at the melting temperature of 0 °c °c. Substances can change state, usually when they are heated or cooled. At the melting point, they have enough kinetic. Melting of ice occurs in two steps:. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the. What Happens When Ice Melts In Terms Of Energy.
From www.youtube.com
Why is the melting of ice a PHYSICAL change? YouTube What Happens When Ice Melts In Terms Of Energy The ice cubes are at the melting temperature of 0 °c °c. Melting of ice occurs in two steps:. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. At the. What Happens When Ice Melts In Terms Of Energy.
From classroom.synonym.com
What Happens When Ice Is Added to Hot Water and How Will the Energy What Happens When Ice Melts In Terms Of Energy Substances can change state, usually when they are heated or cooled. For example, liquid water turns into steam when it is heated enough, and. Heat is transferred from the soda to the ice for melting. The ice cubes are at the melting temperature of 0 °c °c. For solid water (ice), the melting point is 0 °c., the energy gained. What Happens When Ice Melts In Terms Of Energy.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Happens When Ice Melts In Terms Of Energy For example, liquid water turns into steam when it is heated enough, and. At the melting point, they have enough kinetic. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. Using the equation for a change in temperature and the value for water from table 14.7.1. What Happens When Ice Melts In Terms Of Energy.
From www.britannica.com
Ice Definition, Structure, Properties, Freezing Point, & Facts What Happens When Ice Melts In Terms Of Energy For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. Heat is transferred from the soda to the ice for melting. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously.. What Happens When Ice Melts In Terms Of Energy.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens When Ice Melts In Terms Of Energy For example, liquid water turns into steam when it is heated enough, and. The ice cubes are at the melting temperature of 0 °c °c. Melting of ice occurs in two steps:. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. As the. What Happens When Ice Melts In Terms Of Energy.
From www.artofit.org
Melting term understand ice melt work sheet print Artofit What Happens When Ice Melts In Terms Of Energy As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. Using the equation for a change in temperature and the value. What Happens When Ice Melts In Terms Of Energy.
From www.slideserve.com
PPT What happens when ICE melts? PowerPoint Presentation, free What Happens When Ice Melts In Terms Of Energy Substances can change state, usually when they are heated or cooled. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. Melting of ice occurs in two steps:. The ice cubes are at the melting temperature of 0 °c °c. For solid water (ice), the melting point. What Happens When Ice Melts In Terms Of Energy.
From www.scribd.com
What Happens When Ice Melts PDF What Happens When Ice Melts In Terms Of Energy Melting of ice occurs in two steps:. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. For solid water (ice), the melting point is 0 °c., the energy gained by the particles allows them to partly overcome the strong forces holding them. At the melting point,. What Happens When Ice Melts In Terms Of Energy.
From www.fity.club
Melting Ice Cubes Diagram What Happens When Ice Melts In Terms Of Energy For example, liquid water turns into steam when it is heated enough, and. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. Heat is transferred from the soda to the ice for melting. For solid water (ice), the melting. What Happens When Ice Melts In Terms Of Energy.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Ice's Thermal What Happens When Ice Melts In Terms Of Energy Substances can change state, usually when they are heated or cooled. At the melting point, they have enough kinetic. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. 1,. What Happens When Ice Melts In Terms Of Energy.
From study.com
Polar Ice Caps Melting Causes & Impacts Lesson What Happens When Ice Melts In Terms Of Energy Substances can change state, usually when they are heated or cooled. Using the equation for a change in temperature and the value for water from table 14.7.1 14.7. Heat is transferred from the soda to the ice for melting. At the melting point, they have enough kinetic. 1, we find that q = mlf = (1.0 kg)(334 kj/kg) = 334.. What Happens When Ice Melts In Terms Of Energy.