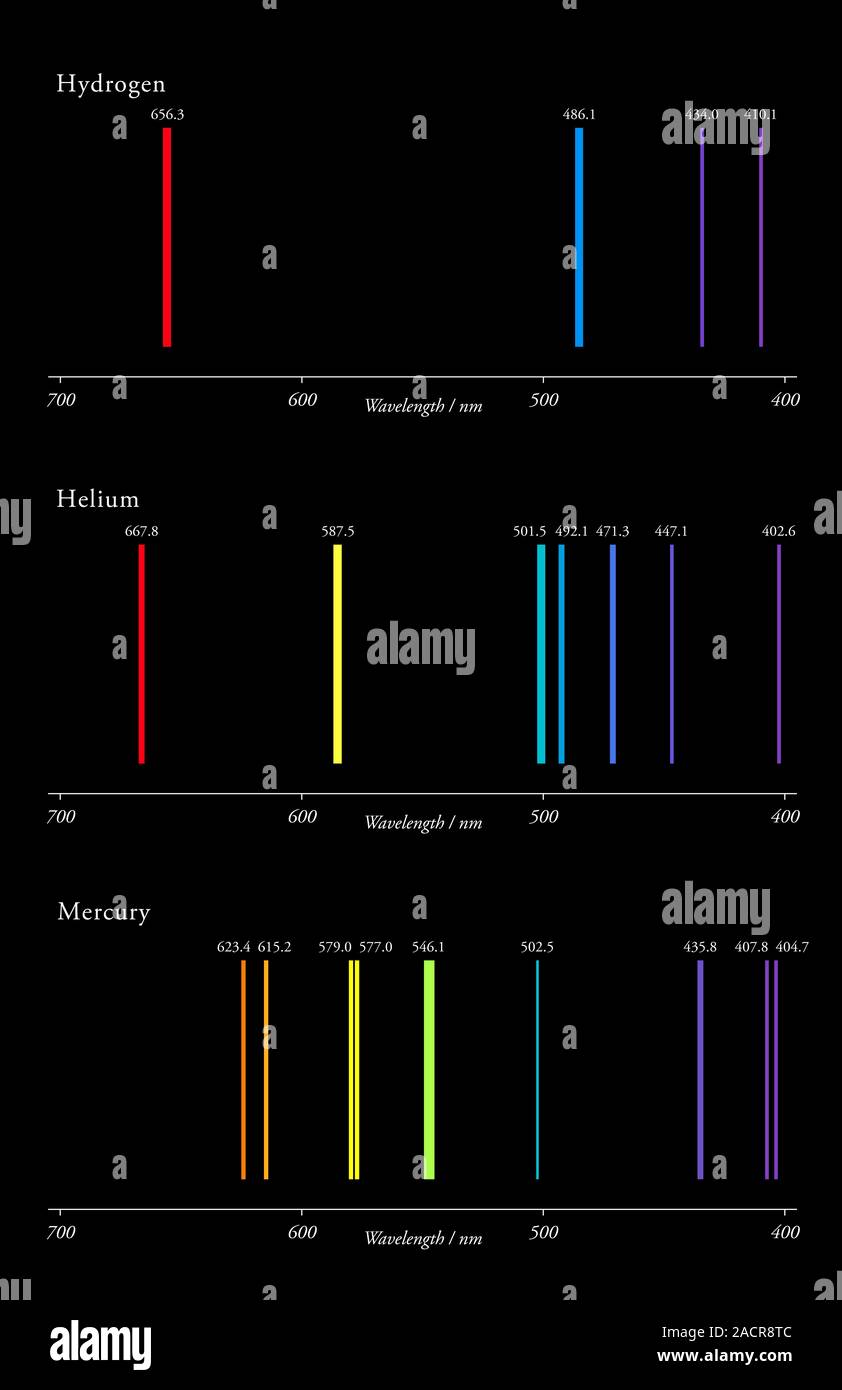
Emission Spectra Colors . Absorption spectra are lit with dark bands; The set of individual colors emitted by an element is called its spectrum. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Every atomic element has a unique absorption and emission spectrum. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectra of various atoms. However, when separated using a prism or diffraction grating, the. Emission spectra are dark with lit. Four distinct colored lines can be perceived if the light is viewed through a spectroscope. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The spectroscope separates the light of varying. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.
from www.alamy.com
Emission spectra are dark with lit. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. The spectroscope separates the light of varying. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. However, when separated using a prism or diffraction grating, the. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Every atomic element has a unique absorption and emission spectrum. The emission spectra of various atoms. Absorption spectra are lit with dark bands;
HHeHg emission spectra. Graphical representation of the emission
Emission Spectra Colors The spectroscope separates the light of varying. The set of individual colors emitted by an element is called its spectrum. Emission spectra are dark with lit. The spectroscope separates the light of varying. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Four distinct colored lines can be perceived if the light is viewed through a spectroscope. However, when separated using a prism or diffraction grating, the. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Absorption spectra are lit with dark bands; Every atomic element has a unique absorption and emission spectrum. The emission spectra of various atoms. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Colors The emission spectra of various atoms. Four distinct colored lines can be perceived if the light is viewed through a spectroscope. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The set of individual colors emitted by an element is called its spectrum. Emission and absorption spectra form. Emission Spectra Colors.
From sciencephotogallery.com
Flame Emission Spectra Of Alkali Metals by Science Photo Library Emission Spectra Colors The emission spectra of various atoms. The set of individual colors emitted by an element is called its spectrum. Absorption spectra are lit with dark bands; The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Four distinct colored lines can be perceived if. Emission Spectra Colors.
From www.pinterest.com
emission spectra and energy levels Learn physics, Physics classroom Emission Spectra Colors Absorption spectra are lit with dark bands; The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Every atomic element has a unique absorption and emission spectrum. When hydrogen gas is placed into a tube and electric current passed through it, the color of. Emission Spectra Colors.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra Colors The set of individual colors emitted by an element is called its spectrum. Four distinct colored lines can be perceived if the light is viewed through a spectroscope. The spectroscope separates the light of varying. Every atomic element has a unique absorption and emission spectrum. Emission spectra are dark with lit. However, when separated using a prism or diffraction grating,. Emission Spectra Colors.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Colors Emission spectra are dark with lit. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Absorption spectra are lit with dark bands; Four distinct colored lines can be perceived if the light is. Emission Spectra Colors.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra Colors However, when separated using a prism or diffraction grating, the. Every atomic element has a unique absorption and emission spectrum. Absorption spectra are lit with dark bands; The emission spectra of various atoms. Emission spectra are dark with lit. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink.. Emission Spectra Colors.
From www.researchgate.net
Emission spectra of different light sources (a) incandescent tungsten Emission Spectra Colors The set of individual colors emitted by an element is called its spectrum. Absorption spectra are lit with dark bands; However, when separated using a prism or diffraction grating, the. Every atomic element has a unique absorption and emission spectrum. Emission spectra are dark with lit. The emission spectra of various atoms. Since the spectrum of each element is unique,. Emission Spectra Colors.
From www.alamy.com
Light spectrum color wavelength radiation prism line Emission Spectra Colors Emission spectra are dark with lit. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted. Emission Spectra Colors.
From www.researchgate.net
(a) Optical emission spectra from Nickel measured by high resolution Emission Spectra Colors Emission spectra are dark with lit. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Absorption spectra are lit with dark bands; The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.. Emission Spectra Colors.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Colors Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The set of individual colors emitted by an element is called its spectrum. Absorption spectra are lit with dark bands; Four distinct colored. Emission Spectra Colors.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Emission Spectra Colors Emission spectra are dark with lit. The spectroscope separates the light of varying. Every atomic element has a unique absorption and emission spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emission spectra of various atoms. When hydrogen gas is placed. Emission Spectra Colors.
From www.researchgate.net
a emission spectra; b normalized emission spectra; c UV spectrum (left Emission Spectra Colors When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Absorption spectra are lit with dark bands; The spectroscope separates the light of varying. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. The set of individual colors emitted by an element. Emission Spectra Colors.
From www.sciencephoto.com
HHeHg emission spectra Stock Image C017/7260 Science Photo Library Emission Spectra Colors The spectroscope separates the light of varying. Absorption spectra are lit with dark bands; The emission spectra of various atoms. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Emission spectra are dark with lit. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure. Emission Spectra Colors.
From www.slideserve.com
PPT EMISSION SPECTRUM PowerPoint Presentation, free download ID5777174 Emission Spectra Colors Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The emission spectra of various atoms. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. However, when separated using a prism or diffraction grating, the. Every atomic element has a unique absorption and. Emission Spectra Colors.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra Colors The spectroscope separates the light of varying. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. The emission spectra of various atoms. Emission spectra are dark with lit. The set of individual. Emission Spectra Colors.
From www.alamy.com
HHeHg emission spectra. Graphical representation of the emission Emission Spectra Colors The emission spectra of various atoms. Absorption spectra are lit with dark bands; Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The set of individual colors emitted by an element is called its spectrum. Emission spectra are dark with lit. However, when separated using a prism or diffraction. Emission Spectra Colors.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Colors Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Emission spectra are dark with lit. The set of individual colors emitted by an element is called its spectrum. Four distinct colored lines can be perceived if the light is viewed through a spectroscope. The emission spectrum (or line spectrum) of a chemical element. Emission Spectra Colors.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Colors However, when separated using a prism or diffraction grating, the. Emission spectra are dark with lit. The set of individual colors emitted by an element is called its spectrum. Absorption spectra are lit with dark bands; Four distinct colored lines can be perceived if the light is viewed through a spectroscope. When hydrogen gas is placed into a tube and. Emission Spectra Colors.
From www.expii.com
Atomic Spectra — Overview & Application Expii Emission Spectra Colors Four distinct colored lines can be perceived if the light is viewed through a spectroscope. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Emission Spectra Colors.
From pages.uoregon.edu
Galaxies Emission Spectra Colors When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Emission spectra are dark with lit. Absorption spectra are lit with dark bands; Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. The emission spectrum (or line spectrum) of a chemical element. Emission Spectra Colors.
From www.researchgate.net
Emission spectra of LEDs with different colors. Download Scientific Emission Spectra Colors However, when separated using a prism or diffraction grating, the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Every atomic element has a unique absorption and emission spectrum. Absorption spectra are lit with dark bands; Emission and absorption spectra form the basis. Emission Spectra Colors.
From winstonmcyponce.blogspot.com
Atomic Emission Spectrum of Hydrogen WinstonmcyPonce Emission Spectra Colors Four distinct colored lines can be perceived if the light is viewed through a spectroscope. The spectroscope separates the light of varying. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectra of various atoms. The emission spectrum (or line spectrum) of a chemical element is. Emission Spectra Colors.
From www.sciencephoto.com
Emission spectrum of mercury Stock Image A150/0022 Science Photo Emission Spectra Colors The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Absorption spectra are lit with dark bands; Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. When hydrogen gas is placed into a tube and electric current. Emission Spectra Colors.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Colors The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. However, when separated using a prism or diffraction grating, the. The emission spectra of. Emission Spectra Colors.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Emission Spectra Colors The spectroscope separates the light of varying. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Every atomic element has a unique absorption and emission spectrum. Four. Emission Spectra Colors.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Emission Spectra Colors The spectroscope separates the light of varying. The emission spectra of various atoms. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.. Emission Spectra Colors.
From sciencephotogallery.com
Hydrogen And Helium Spectra by Science Photo Library Emission Spectra Colors The set of individual colors emitted by an element is called its spectrum. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Absorption spectra are lit with dark bands; When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Every atomic element. Emission Spectra Colors.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Spectra Colors The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectra of various atoms. The set of individual colors emitted by. Emission Spectra Colors.
From www.researchgate.net
Emission spectra of LED light sources. Download Scientific Diagram Emission Spectra Colors Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The set of individual colors emitted by an element is called its spectrum. The emission spectrum (or line spectrum) of a chemical element. Emission Spectra Colors.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectra Colors Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Emission Spectra Colors.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Emission Spectra Colors The set of individual colors emitted by an element is called its spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Every atomic element has a unique absorption and emission spectrum. However, when separated using a prism or diffraction grating, the. Since. Emission Spectra Colors.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Emission Spectra Colors The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The set of individual colors emitted by an element is called its spectrum. Emission spectra are dark with lit. When hydrogen gas is placed into a tube and electric current passed through it, the. Emission Spectra Colors.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Emission Spectra Colors Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Absorption spectra are lit with dark bands; When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Emission spectra are dark with lit. The set of individual colors emitted. Emission Spectra Colors.
From medium.com
Light and Dark Understanding Spectrometry by Amelia Settembre Medium Emission Spectra Colors The set of individual colors emitted by an element is called its spectrum. Emission spectra are dark with lit. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the.. Emission Spectra Colors.
From www.youtube.com
Emission spectrum of hydrogen Chemistry Khan Academy YouTube Emission Spectra Colors Emission spectra are dark with lit. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The spectroscope separates the light of varying. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.. Emission Spectra Colors.