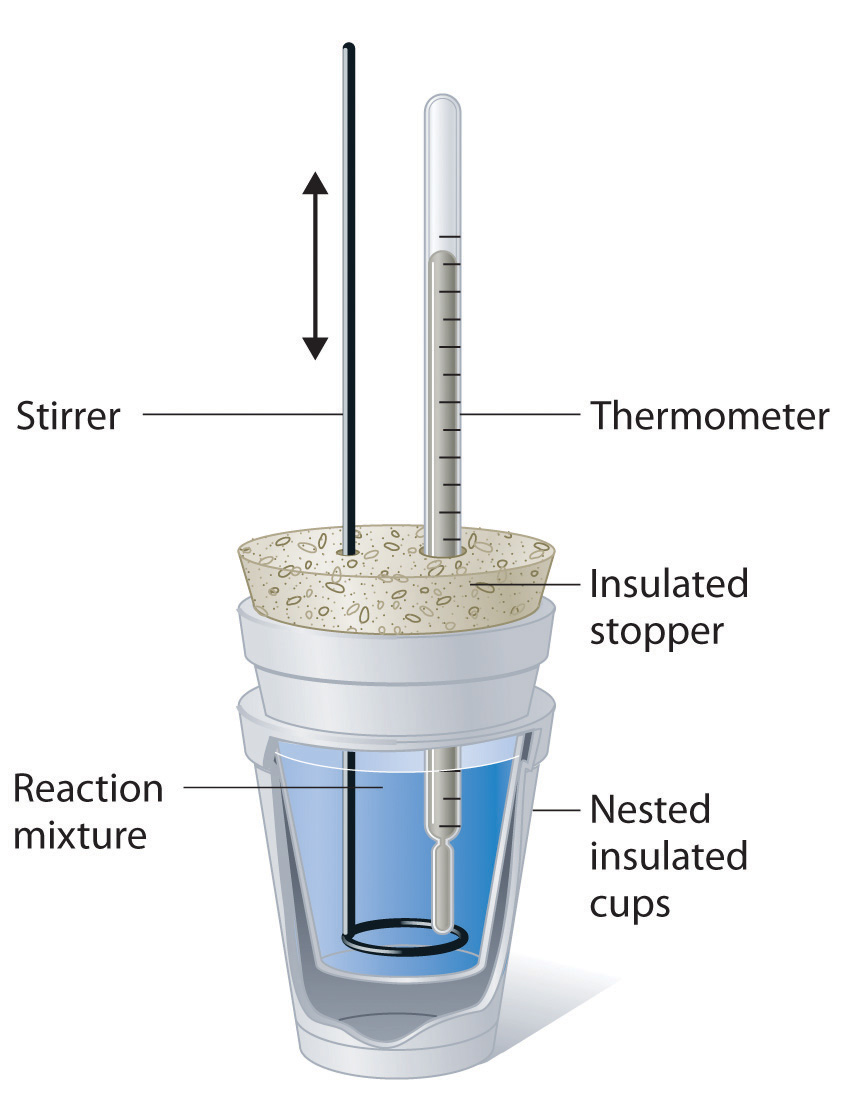
Figure Of Calorimeter . For example, when an exothermic reaction occurs in solution in a calorimeter, the. Explain the technique of calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Calculate and interpret heat and. Calculate and interpret heat and related properties using typical calorimetry data. By the end of this section, you will be able to: Explain the technique of calorimetry. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings.
from users.highland.edu
This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). By the end of this section, you will be able to: Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Explain the technique of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explain the technique of calorimetry.
Calorimetry
Figure Of Calorimeter For example, when an exothermic reaction occurs in solution in a calorimeter, the. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate and interpret heat and related properties using typical calorimetry data. For example, when an exothermic reaction occurs in solution in a calorimeter, the. By the end of this section, you will be able to: Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Explain the technique of calorimetry. Explain the technique of calorimetry. Calculate and interpret heat and.
From www.studypool.com
SOLUTION What is glass calorimeter explain with diagram and example Figure Of Calorimeter Explain the technique of calorimetry. Calculate and interpret heat and. By the end of this section, you will be able to: Calculate and interpret heat and related properties using typical calorimetry data. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving. Figure Of Calorimeter.
From schoolbag.info
Thermodynamics Review the Knowledge You Need to Score High 5 Steps Figure Of Calorimeter Calculate and interpret heat and related properties using typical calorimetry data. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. This type of calorimeter consists of a robust steel container (the. Figure Of Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry Figure Of Calorimeter Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. Explain the technique of calorimetry. By. Figure Of Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Figure Of Calorimeter Explain the technique of calorimetry. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Figure Of Calorimeter.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Figure Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate and interpret heat and. Calculate and interpret heat and related properties using typical calorimetry data. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). Explain. Figure Of Calorimeter.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Figure Of Calorimeter Explain the technique of calorimetry. Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Explain the technique of calorimetry. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the. Figure Of Calorimeter.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Figure Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). By the end of this section, you will be able to: Figure \ (\pageindex {1}\) shows the. Figure Of Calorimeter.
From www.researchgate.net
Adiabatic calorimeter (a) schematic layout of calorimeter, ice Figure Of Calorimeter For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Calculate and interpret heat and related properties using typical calorimetry data. By the end of this section, you will be able to: Calculate and interpret heat. Figure Of Calorimeter.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Figure Of Calorimeter Explain the technique of calorimetry. Calculate and interpret heat and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). Figure \ (\pageindex {1}\) shows the basic. Figure Of Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Figure Of Calorimeter Calculate and interpret heat and. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. For example, when an exothermic reaction occurs in solution in. Figure Of Calorimeter.
From www.animalia-life.club
Calorimeter Diagram Figure Of Calorimeter This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). By the end of this section, you will be able to: Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Calculate and interpret heat. Figure Of Calorimeter.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Figure Of Calorimeter By the end of this section, you will be able to: Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the. Figure Of Calorimeter.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Figure Of Calorimeter Explain the technique of calorimetry. Calculate and interpret heat and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). Figure \ (\pageindex {1}\) shows the basic. Figure Of Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Figure Of Calorimeter Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. By the end of this section, you will be able to: For example, when an exothermic reaction occurs in solution in a. Figure Of Calorimeter.
From saylordotorg.github.io
Calorimetry Figure Of Calorimeter Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explain the technique of calorimetry. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray. Figure Of Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry I Figure Of Calorimeter Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. By the end of this section, you will be able to: Explain the technique of calorimetry. Explain the technique of calorimetry. For. Figure Of Calorimeter.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Figure Of Calorimeter Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. By the end of this section, you will be able to: Calculate and interpret heat and related properties using typical calorimetry data.. Figure Of Calorimeter.
From www.animalia-life.club
Calorimeter Diagram Figure Of Calorimeter By the end of this section, you will be able to: Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Explain the technique of calorimetry. Explain the technique of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Figure \ (\pageindex {1}\) shows. Figure Of Calorimeter.
From www.researchgate.net
Schematic representation of calorimeter system US4 at SKINR. Download Figure Of Calorimeter Calculate and interpret heat and. Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explain the. Figure Of Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Figure Of Calorimeter Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By the end of this section, you will be able to: This type of calorimeter consists of a robust steel. Figure Of Calorimeter.
From www.researchgate.net
1 Schematic diagram of calorimeter. (From Viranichai, S., Effect of Figure Of Calorimeter Explain the technique of calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Calculate and interpret heat and related properties using typical calorimetry data. For example, when an. Figure Of Calorimeter.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Figure Of Calorimeter Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. By the end of this section, you will be able to: Explain the technique of calorimetry. Explain the technique of calorimetry. For. Figure Of Calorimeter.
From quizlet.com
Sketch a simple calorimeter and label the purpose of each co Quizlet Figure Of Calorimeter Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate and interpret heat and. Explain the technique of calorimetry. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the. Figure Of Calorimeter.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Figure Of Calorimeter Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. Explain the technique of calorimetry. Explain the technique of calorimetry. By the end of this section, you will be able to: For. Figure Of Calorimeter.
From users.highland.edu
Calorimetry Figure Of Calorimeter Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of calorimetry. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). Calculate and interpret heat and. Explain the technique of calorimetry. For example, when an exothermic reaction occurs in solution in. Figure Of Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Figure Of Calorimeter Explain the technique of calorimetry. Calculate and interpret heat and. By the end of this section, you will be able to: This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.2.7). A calorimeter is a device used to measure the amount of heat involved in a. Figure Of Calorimeter.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Figure Of Calorimeter Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. By the end of this section, you will be able to: Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. For example, when an exothermic reaction occurs in solution in a calorimeter,. Figure Of Calorimeter.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement Figure Of Calorimeter By the end of this section, you will be able to: Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. This type of calorimeter consists of a robust steel container (the. Figure Of Calorimeter.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure Figure Of Calorimeter By the end of this section, you will be able to: For example, when an exothermic reaction occurs in solution in a calorimeter, the. Explain the technique of calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a science where you try to find the heat transfer. Figure Of Calorimeter.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Figure Of Calorimeter For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By the end of this section, you will be able to: Calculate and interpret heat and related properties using typical calorimetry data. Figure \ (\pageindex {1}\) shows the. Figure Of Calorimeter.
From www.researchgate.net
Technological description of the calorimeter prototype Download Figure Of Calorimeter Explain the technique of calorimetry. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase. Figure Of Calorimeter.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Figure Of Calorimeter Explain the technique of calorimetry. Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the. Figure Of Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Figure Of Calorimeter Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Calculate and interpret heat and related properties using typical calorimetry data. By the end of this section, you will be able to: Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box. Figure Of Calorimeter.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Figure Of Calorimeter Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. Calorimetry is a science where you try to find the heat transfer during a chemical reaction, phase transition, or temperature change. Calculate. Figure Of Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Figure Of Calorimeter Explain the technique of calorimetry. Explain the technique of calorimetry. Figure \ (\pageindex {1}\) shows the basic layout of an ideal calorimeter where the gray shaded box acts as a surface that prevent any heat entering or leaving the solution, and so the solution functions as the surroundings. A calorimeter is a device used to measure the amount of heat. Figure Of Calorimeter.