Catalysts Accelerate A Reaction By Quizlet . It assumes familiarity with basic concepts in the collision. They lower the overall free energy change of the reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; how do catalysts work to accelerate a chemical reaction? the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. catalysts accelerate both the forward and reverse reactions by the same amount. this page explains how adding a catalyst affects the rate of a reaction. On the other hand, the catalysts.
from www.studocu.com
study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. It assumes familiarity with basic concepts in the collision. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They lower the overall free energy change of the reaction. this page explains how adding a catalyst affects the rate of a reaction. On the other hand, the catalysts. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. catalysts accelerate both the forward and reverse reactions by the same amount. the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst.
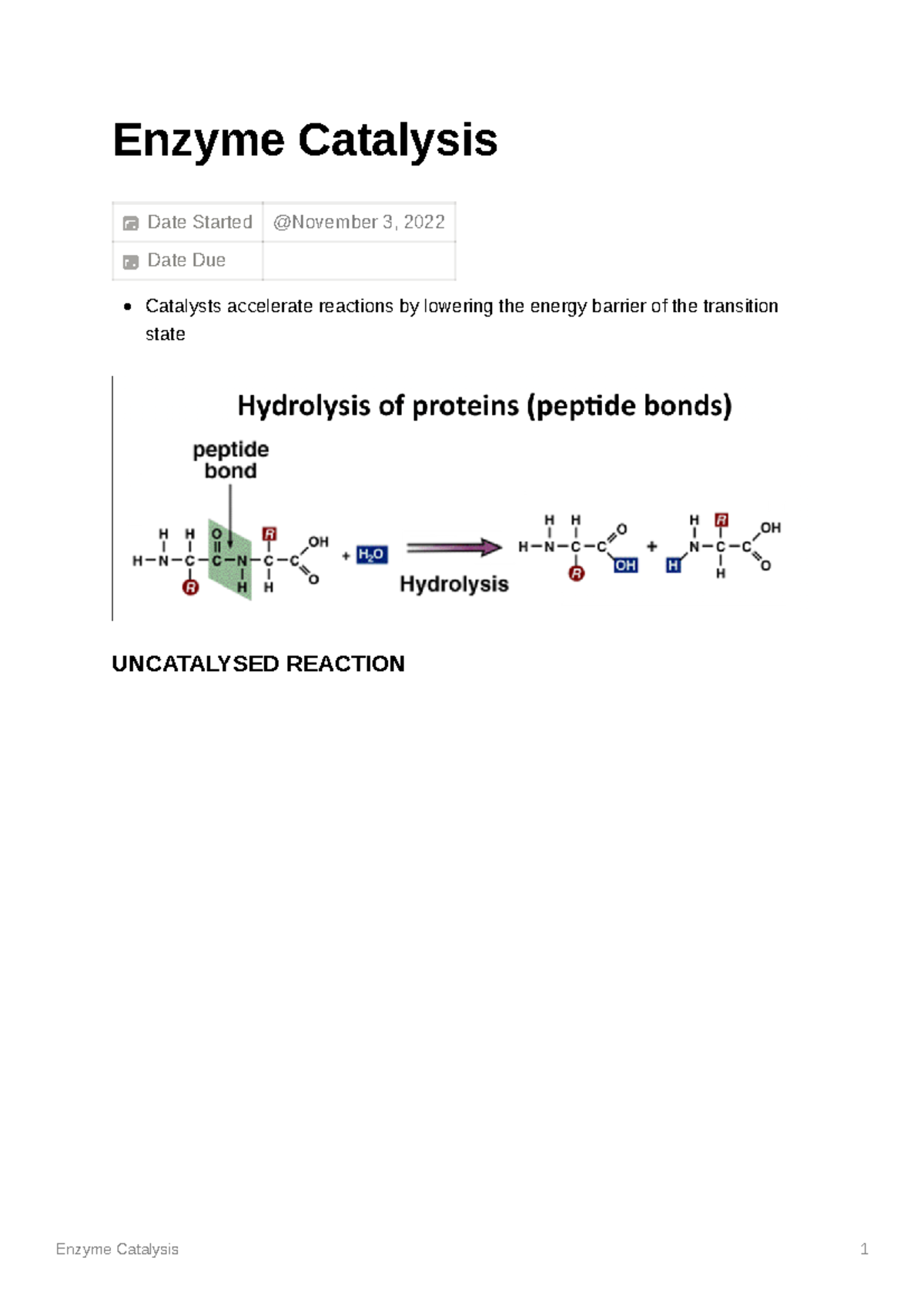
MEDC0003 Enzyme Catalysis Enzyme Catalysis Date Started Date Due
Catalysts Accelerate A Reaction By Quizlet the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; how do catalysts work to accelerate a chemical reaction? They lower the overall free energy change of the reaction. On the other hand, the catalysts. catalysts accelerate both the forward and reverse reactions by the same amount. this page explains how adding a catalyst affects the rate of a reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; It assumes familiarity with basic concepts in the collision. study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst.
From www.chegg.com
Solved 15) Catalysts accelerate a reaction by A) raising the Catalysts Accelerate A Reaction By Quizlet the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts accelerate both the forward and reverse. Catalysts Accelerate A Reaction By Quizlet.
From quizlet.com
Chapter 8 Enzymes Basic Concepts and Diagram Quizlet Catalysts Accelerate A Reaction By Quizlet catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts accelerate both the forward and reverse reactions by the same amount. On the other hand, the catalysts. this page explains how adding a catalyst affects the rate of a reaction. study with quizlet and memorize flashcards containing terms like. Catalysts Accelerate A Reaction By Quizlet.
From www.numerade.com
SOLVEDCatalysts accelerate chemical reactions by lowering the Catalysts Accelerate A Reaction By Quizlet study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. this page explains how adding. Catalysts Accelerate A Reaction By Quizlet.
From slideplayer.com
CHAPTER 9 Chemistry ppt download Catalysts Accelerate A Reaction By Quizlet how do catalysts work to accelerate a chemical reaction? the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. this page explains how adding a catalyst affects the rate of a reaction. catalysts accelerate both the forward and reverse reactions by the same amount. catalysts allow a reaction to. Catalysts Accelerate A Reaction By Quizlet.
From slideplayer.com
University of Louisiana at Lafayette ppt download Catalysts Accelerate A Reaction By Quizlet catalysts accelerate both the forward and reverse reactions by the same amount. They lower the overall free energy change of the reaction. It assumes familiarity with basic concepts in the collision. study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. catalysts allow a reaction to proceed via a pathway. Catalysts Accelerate A Reaction By Quizlet.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysts Accelerate A Reaction By Quizlet the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. They lower the overall free energy change of the reaction. It assumes familiarity with basic concepts in the collision. catalyst, in chemistry, any substance. Catalysts Accelerate A Reaction By Quizlet.
From exorsjyuc.blob.core.windows.net
Catalyst Reaction Meaning at Jarred Mikula blog Catalysts Accelerate A Reaction By Quizlet study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. catalysts accelerate both the forward and reverse reactions by the same amount. They lower the overall free energy change of the reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; this. Catalysts Accelerate A Reaction By Quizlet.
From slideplayer.com
Chapter ppt download Catalysts Accelerate A Reaction By Quizlet They lower the overall free energy change of the reaction. It assumes familiarity with basic concepts in the collision. catalysts accelerate both the forward and reverse reactions by the same amount. the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. catalysts allow a reaction to proceed via a pathway that. Catalysts Accelerate A Reaction By Quizlet.
From stock.adobe.com
Lock and Key Mechanism of Enzymes. Catalysts accelerate chemical Catalysts Accelerate A Reaction By Quizlet It assumes familiarity with basic concepts in the collision. this page explains how adding a catalyst affects the rate of a reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. the most. Catalysts Accelerate A Reaction By Quizlet.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalysts Accelerate A Reaction By Quizlet catalysts accelerate both the forward and reverse reactions by the same amount. the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. this page explains how adding a catalyst affects the rate of a reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy. Catalysts Accelerate A Reaction By Quizlet.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalysts Accelerate A Reaction By Quizlet the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. how do catalysts work to accelerate a. Catalysts Accelerate A Reaction By Quizlet.
From www.slideserve.com
PPT The changes in energy that occur during chemical reactions can Catalysts Accelerate A Reaction By Quizlet the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. catalysts accelerate both the forward and reverse reactions by the same amount. It assumes familiarity with basic concepts in the collision. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. the. Catalysts Accelerate A Reaction By Quizlet.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalysts Accelerate A Reaction By Quizlet catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. On the other hand, the catalysts. It assumes familiarity with basic concepts in the collision. how do catalysts work to accelerate a chemical reaction? the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; . Catalysts Accelerate A Reaction By Quizlet.
From www.nagwa.com
Question Video Identifying the Reason Why Catalysts Are Used in Catalysts Accelerate A Reaction By Quizlet how do catalysts work to accelerate a chemical reaction? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. the ones which accelerate the rate of a chemical reaction is referred to. Catalysts Accelerate A Reaction By Quizlet.
From slideplayer.com
MiniUnit (Chapter 17) ppt download Catalysts Accelerate A Reaction By Quizlet this page explains how adding a catalyst affects the rate of a reaction. how do catalysts work to accelerate a chemical reaction? study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. On the other hand, the catalysts. catalysts allow a reaction to proceed via a pathway that has. Catalysts Accelerate A Reaction By Quizlet.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalysts Accelerate A Reaction By Quizlet catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. this page explains how adding a catalyst affects the rate of a reaction. the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. the most effective catalyst of all is the enzyme. Catalysts Accelerate A Reaction By Quizlet.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube Catalysts Accelerate A Reaction By Quizlet catalysts accelerate both the forward and reverse reactions by the same amount. study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. On the other hand, the catalysts. They lower the overall free energy change of the reaction. this page explains how adding a catalyst affects the rate of a. Catalysts Accelerate A Reaction By Quizlet.
From www.studocu.com
MEDC0003 Enzyme Catalysis Enzyme Catalysis Date Started Date Due Catalysts Accelerate A Reaction By Quizlet how do catalysts work to accelerate a chemical reaction? the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. this page explains how adding a catalyst affects the rate of a reaction. catalysts. Catalysts Accelerate A Reaction By Quizlet.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Accelerate A Reaction By Quizlet catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts accelerate both the forward and reverse reactions by the same amount. how do catalysts work to accelerate a chemical reaction? It assumes familiarity. Catalysts Accelerate A Reaction By Quizlet.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalysts Accelerate A Reaction By Quizlet catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. catalysts accelerate both the forward and reverse reactions by the same amount. They lower the overall free energy change of the reaction. On the other hand, the catalysts. catalyst, in chemistry, any substance that increases the rate of a. Catalysts Accelerate A Reaction By Quizlet.
From slideplayer.com
How Catalysts Work Main Concept ppt download Catalysts Accelerate A Reaction By Quizlet study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. It assumes familiarity with basic concepts in the collision. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; this page explains how adding a catalyst affects the rate of a reaction. On the. Catalysts Accelerate A Reaction By Quizlet.
From www.researchgate.net
The presence of different catalysts (colored asterisks) that accelerate Catalysts Accelerate A Reaction By Quizlet catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They lower the overall free energy change of the reaction. study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. how do catalysts work to accelerate a chemical reaction? this page explains how. Catalysts Accelerate A Reaction By Quizlet.
From www.studocu.com
Enzymes for biology ☘ Enzymes Enzymes are biological catalysts that Catalysts Accelerate A Reaction By Quizlet how do catalysts work to accelerate a chemical reaction? this page explains how adding a catalyst affects the rate of a reaction. They lower the overall free energy change of the reaction. It assumes familiarity with basic concepts in the collision. study with quizlet and memorize flashcards containing terms like the law of conservation of energy states,. Catalysts Accelerate A Reaction By Quizlet.
From slideplayer.com
CHAPTER 9 Chemistry ppt download Catalysts Accelerate A Reaction By Quizlet how do catalysts work to accelerate a chemical reaction? study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts accelerate both the forward and reverse reactions by the same amount. It assumes. Catalysts Accelerate A Reaction By Quizlet.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at Catalysts Accelerate A Reaction By Quizlet catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They lower the overall free energy change of the reaction. study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. catalysts allow a reaction to proceed via a pathway that has a lower activation. Catalysts Accelerate A Reaction By Quizlet.
From www.chegg.com
Solved Question 7 Catalysts accelerate a reaction by O Catalysts Accelerate A Reaction By Quizlet this page explains how adding a catalyst affects the rate of a reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. catalysts allow a reaction to proceed via a pathway that. Catalysts Accelerate A Reaction By Quizlet.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts Accelerate A Reaction By Quizlet this page explains how adding a catalyst affects the rate of a reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. It assumes familiarity with basic concepts in the collision. catalysts accelerate both the forward and reverse reactions by the same amount. On the other hand, the. Catalysts Accelerate A Reaction By Quizlet.
From slideplayer.com
Chapter 6 Lecture Outline ppt download Catalysts Accelerate A Reaction By Quizlet On the other hand, the catalysts. catalysts accelerate both the forward and reverse reactions by the same amount. this page explains how adding a catalyst affects the rate of a reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; It assumes familiarity with basic concepts in the collision. . Catalysts Accelerate A Reaction By Quizlet.
From slideplayer.com
University of Louisiana at Lafayette ppt download Catalysts Accelerate A Reaction By Quizlet catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. this page explains how adding a catalyst affects the rate of a reaction. On the other hand, the catalysts. how do. Catalysts Accelerate A Reaction By Quizlet.
From quizlet.com
catalysts Diagram Quizlet Catalysts Accelerate A Reaction By Quizlet catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts accelerate both the forward and reverse reactions by the same amount. how do catalysts work to accelerate a chemical reaction? the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. study with. Catalysts Accelerate A Reaction By Quizlet.
From www.studocu.com
BIOL 1345 Module 5 Metabolism Enzymes are chemical catalysts that Catalysts Accelerate A Reaction By Quizlet the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. It assumes familiarity with basic concepts in the collision. how do catalysts work to accelerate a chemical reaction? the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; They lower the overall free energy change. Catalysts Accelerate A Reaction By Quizlet.
From www.chegg.com
Solved Question 21 How do catalysts work to accelerate a Catalysts Accelerate A Reaction By Quizlet study with quizlet and memorize flashcards containing terms like the law of conservation of energy states, catalysts. this page explains how adding a catalyst affects the rate of a reaction. the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. the most effective catalyst of all is the enzyme catalase,. Catalysts Accelerate A Reaction By Quizlet.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalysts Accelerate A Reaction By Quizlet It assumes familiarity with basic concepts in the collision. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. On the other hand, the catalysts. catalysts allow a reaction to proceed via a pathway that. Catalysts Accelerate A Reaction By Quizlet.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions Catalysts Accelerate A Reaction By Quizlet catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. the ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. how do catalysts work to accelerate a chemical reaction? On the other hand, the catalysts. They lower the overall free energy change of. Catalysts Accelerate A Reaction By Quizlet.
From slidetodoc.com
Enzymes catalysis Enzyme catalyzed chemical reactions via both Catalysts Accelerate A Reaction By Quizlet They lower the overall free energy change of the reaction. On the other hand, the catalysts. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. this page explains how adding a catalyst affects the rate of a reaction. the ones which accelerate the rate of a chemical reaction. Catalysts Accelerate A Reaction By Quizlet.