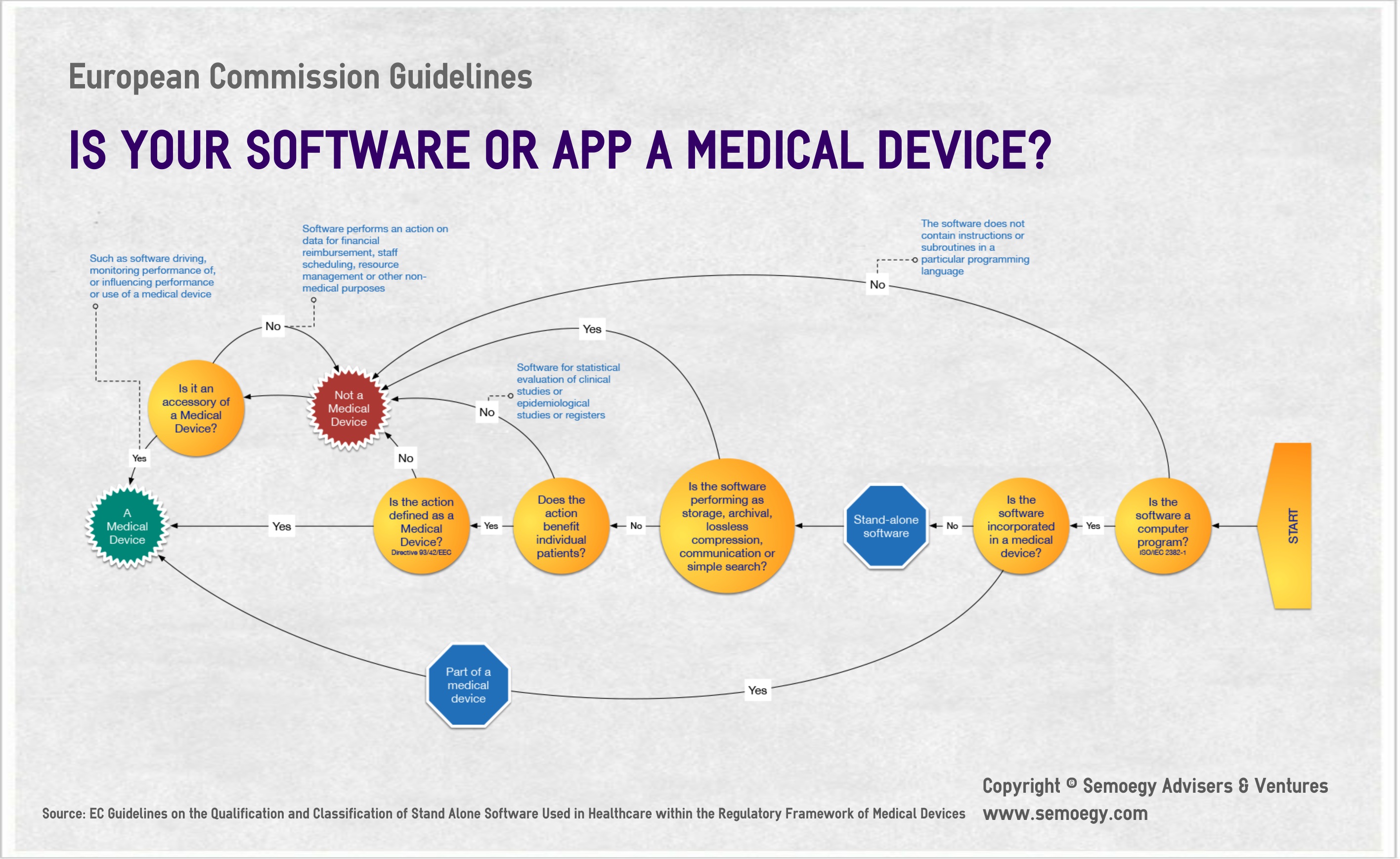
Medical Device Stand-Alone Software Including Apps . Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a.
from semoegy.com
This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in.
Medical Mobile Apps Medical Device Regulations Training Semoegy
Medical Device Stand-Alone Software Including Apps Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'.
From www.medicaltechnologyuk.com
Medical device standalone software including apps (including IVDMDs Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document. Medical Device Stand-Alone Software Including Apps.
From vdocuments.mx
MHRA Software flowchart · flow chart Introduction Medical purpose flow Medical Device Stand-Alone Software Including Apps Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Software as a medical. Medical Device Stand-Alone Software Including Apps.
From pharmacyconsulting.co.uk
Consultancy Archives Pharmacy Consulting Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a.. Medical Device Stand-Alone Software Including Apps.
From podcasts.apple.com
在 Apple Podcasts 上的《Device Advice by RQM+》:RQM+ Live! 61 — Medical Medical Device Stand-Alone Software Including Apps The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. This guidance document. Medical Device Stand-Alone Software Including Apps.
From www.thebalance.com
A Guide to StandAlone Software Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a.. Medical Device Stand-Alone Software Including Apps.
From www.researchgate.net
(PDF) STANDALONE SOFTWARE AS A MEDICAL DEVICE QUALIFICATION AND Medical Device Stand-Alone Software Including Apps The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements. Medical Device Stand-Alone Software Including Apps.
From knobbemedical.com
MHRA Updates Guidance on Healthcare Apps as Medical Devices Knobbe Medical Device Stand-Alone Software Including Apps The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Software as a medical. Medical Device Stand-Alone Software Including Apps.
From www.pdffiller.com
Fillable Online Guidance on medical device standalone software Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. The legislative framework on medical. Medical Device Stand-Alone Software Including Apps.
From benefitintelligence.com
UBA Teladoc Program Benefit Intelligence Medical Device Stand-Alone Software Including Apps Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. This guidance document replaces the previous mhra guidance titled “medical device. Medical Device Stand-Alone Software Including Apps.
From semoegy.com
Medical Mobile Apps Medical Device Regulations Training Semoegy Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Software as a medical. Medical Device Stand-Alone Software Including Apps.
From studylib.net
Medical device standalone software including apps Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a.. Medical Device Stand-Alone Software Including Apps.
From www.slideserve.com
PPT Medical Devices PowerPoint Presentation, free download ID9395135 Medical Device Stand-Alone Software Including Apps Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”.. Medical Device Stand-Alone Software Including Apps.
From www.mastercontrol.com
Medical Device Innovation Manufacturing Quality Data MasterControl Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a.. Medical Device Stand-Alone Software Including Apps.
From www.regdesk.co
MHRA Guidance on Standalone Software Overview RegDesk Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Software as a medical device (samd, being standalone software and software included in wider hardware). Medical Device Stand-Alone Software Including Apps.
From www.slideshare.net
From Servers to Medical Devices Medical Device Stand-Alone Software Including Apps Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Software as a medical. Medical Device Stand-Alone Software Including Apps.
From www.afpharmaservice.com
UPDATED GUIDANCE MHRA Medical device standalone software including Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Software as a medical device (samd, being standalone software and software included in wider hardware). Medical Device Stand-Alone Software Including Apps.
From present5.com
Medical Device Software Quality Assurance and Risk Assessment Medical Device Stand-Alone Software Including Apps The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Software as a. Medical Device Stand-Alone Software Including Apps.
From www.fladgate.com
COVID19 regulation of software including apps Fladgate Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a.. Medical Device Stand-Alone Software Including Apps.
From www.scribd.com
Medical Device StandAlone Software Including Apps PDF Medical Medical Device Stand-Alone Software Including Apps Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as. Medical Device Stand-Alone Software Including Apps.
From www.researchgate.net
(PDF) Medical app minefield radiologists use of medical apps for Medical Device Stand-Alone Software Including Apps The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. This guidance document. Medical Device Stand-Alone Software Including Apps.
From uni-cert.ua
Standalone software as a medical device Features of its Medical Device Stand-Alone Software Including Apps Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. This guidance document replaces. Medical Device Stand-Alone Software Including Apps.
From www.tentamus.com
Can a Software be a medical device? Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. This guidance document replaces the previous mhra guidance titled “medical device. Medical Device Stand-Alone Software Including Apps.
From www.slideserve.com
PPT Medical Devices PowerPoint Presentation, free download ID9395135 Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. The legislative framework on medical devices has been revised and 2 new. Medical Device Stand-Alone Software Including Apps.
From 4easyreg.com
IEC 823041 and its Application for StandAlone Software Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Software as a. Medical Device Stand-Alone Software Including Apps.
From semoegy.com
Medical Mobile Apps Medical Device Regulations Training Semoegy Medical Device Stand-Alone Software Including Apps Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. The legislative framework on medical devices has been revised and 2 new. Medical Device Stand-Alone Software Including Apps.
From www.medicept.com
MHRA Standalone Software and Apps MEDIcept Medical Device Stand-Alone Software Including Apps Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. The legislative framework on medical devices has been revised and 2 new regulations will become. Medical Device Stand-Alone Software Including Apps.
From www.trademed.com
StandAlone System The AccuGold+ Touch Medical Equipment and Medical Device Stand-Alone Software Including Apps Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. The legislative framework on medical devices has been revised and 2 new. Medical Device Stand-Alone Software Including Apps.
From www.slideshare.net
IQ Messenger Whitepaper CE MDD Medical Device Stand-Alone Software Including Apps Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”.. Medical Device Stand-Alone Software Including Apps.
From www.researchgate.net
(PDF) CHALLENGES OF CLASSIFICATION OF STANDALONE SOFTWARE AS A MEDICAL Medical Device Stand-Alone Software Including Apps Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”.. Medical Device Stand-Alone Software Including Apps.
From www.qualitymeddev.com
IEC 823041 and its Application for StandAlone Software Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. The. Medical Device Stand-Alone Software Including Apps.
From www.slideserve.com
PPT Medical Devices PowerPoint Presentation, free download ID9395135 Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. Standalone software and apps that meet the definition of a medical device. Medical Device Stand-Alone Software Including Apps.
From www.regdesk.co
MHRA Guidance on Standalone Software Overview RegDesk Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a.. Medical Device Stand-Alone Software Including Apps.
From www.slideshare.net
UK MHRA Guidance on Medical Device StandAlone software including Apps Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in.. Medical Device Stand-Alone Software Including Apps.
From medenvoyglobal.com
Medical Device StandAlone Software Under the UK MHRA Medical Device Stand-Alone Software Including Apps This guidance document replaces the previous mhra guidance 'medical device standalone software, including apps'. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements in. This guidance document replaces the previous mhra guidance titled “medical device standalone software, including apps”. The legislative framework on medical. Medical Device Stand-Alone Software Including Apps.
From semoegy.com
Medical Mobile Apps Medical Device Regulations Training Semoegy Medical Device Stand-Alone Software Including Apps The legislative framework on medical devices has been revised and 2 new regulations will become progressively. Software as a medical device (samd, being standalone software and software included in wider hardware) (including ai as a. Standalone software and apps that meet the definition of a medical device are required to be ce marked in accordance with the eu regulatory requirements. Medical Device Stand-Alone Software Including Apps.