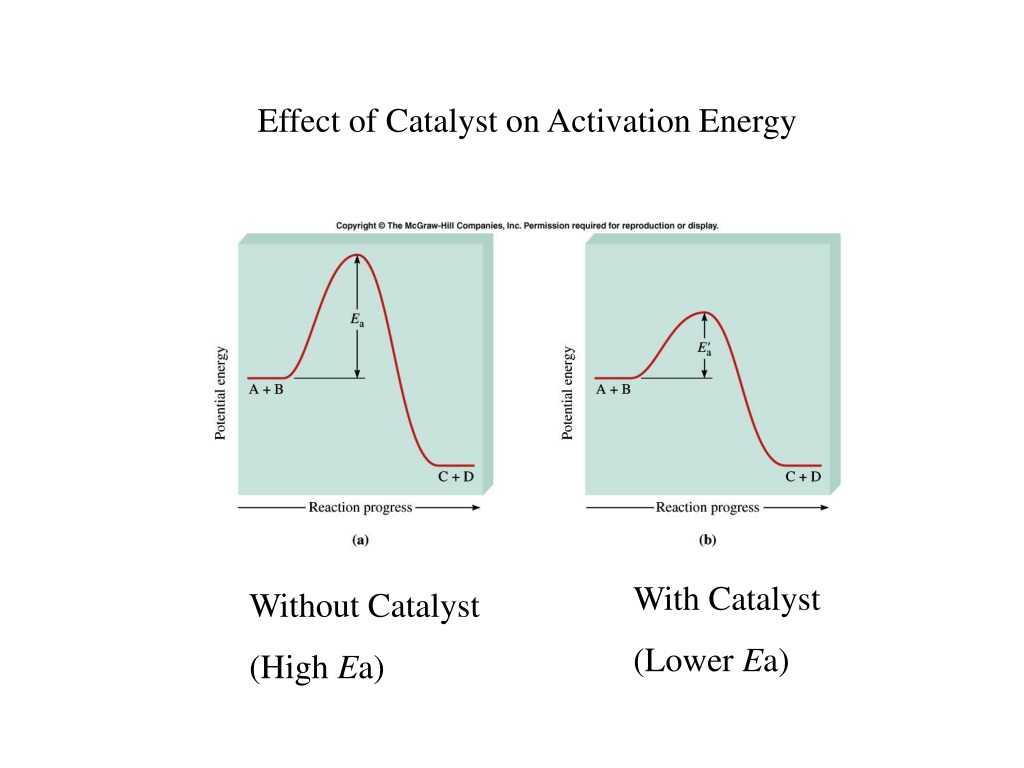
Why Catalyst Decrease Activation Energy . in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. a catalyst provides an alternative route for the reaction with a lower activation energy. Catalysts are not consumed by the reaction. It does not lower the activation energy of the reaction. A catalyst provides an alternative route for the reaction. catalysts increase the rate of reaction. A catalyst lowers the activation energy of a chemical reaction. adding a catalyst has exactly this effect of shifting the activation energy. That alternative route has a lower. effect of enzymes and catalysts. Enzymes are examples of catalysts. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. A small quantity of catalyst should be able to.
from www.slideserve.com
catalysts increase the rate of reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. That alternative route has a lower. A catalyst lowers the activation energy of a chemical reaction. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. adding a catalyst has exactly this effect of shifting the activation energy. effect of enzymes and catalysts. Enzymes are examples of catalysts. It does not lower the activation energy of the reaction. Catalysts are not consumed by the reaction.
PPT Chemical Change Energy, Rate and Equilibrium Thermodynamics
Why Catalyst Decrease Activation Energy adding a catalyst has exactly this effect of shifting the activation energy. adding a catalyst has exactly this effect of shifting the activation energy. Catalysts are not consumed by the reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the activation energy of the reaction. catalysts increase the rate of reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. That alternative route has a lower. effect of enzymes and catalysts. Enzymes are examples of catalysts. A catalyst provides an alternative route for the reaction. A small quantity of catalyst should be able to. A catalyst lowers the activation energy of a chemical reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Why Catalyst Decrease Activation Energy in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. It does not lower the activation energy of the reaction. That alternative route has a lower. catalysts increase. Why Catalyst Decrease Activation Energy.
From brainly.com
Classify each statement about catalysts as true or false. Catalyst Why Catalyst Decrease Activation Energy in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. effect of enzymes and catalysts. A catalyst provides an alternative route for the reaction. adding a catalyst has exactly this effect of shifting the activation energy. That alternative route has a lower. a catalyst. Why Catalyst Decrease Activation Energy.
From ceenbrfk.blob.core.windows.net
Catalysts Lower The Activation Energy Required For A Reaction at Elnora Why Catalyst Decrease Activation Energy A catalyst lowers the activation energy of a chemical reaction. That alternative route has a lower. It does not lower the activation energy of the reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. adding a catalyst has exactly this effect of shifting the. Why Catalyst Decrease Activation Energy.
From quizosteopathy.z4.web.core.windows.net
What Lowers Activation Energy Why Catalyst Decrease Activation Energy A small quantity of catalyst should be able to. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. Catalysts are not consumed by the reaction. It does not lower the activation energy of the reaction. adding a catalyst has exactly this effect of shifting the. Why Catalyst Decrease Activation Energy.
From www.slideserve.com
PPT Chemical Change Energy, Rate and Equilibrium Thermodynamics Why Catalyst Decrease Activation Energy catalysts increase the rate of reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. Catalysts are not consumed by the reaction. a catalyst is able to reduce the. Why Catalyst Decrease Activation Energy.
From guidelibdiapedetic.z22.web.core.windows.net
Catalyst Diagram Chemistry Why Catalyst Decrease Activation Energy A small quantity of catalyst should be able to. It does not lower the activation energy of the reaction. A catalyst provides an alternative route for the reaction. effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. a catalyst is able to reduce the activation energy by forming a transition state in. Why Catalyst Decrease Activation Energy.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation ID5736986 Why Catalyst Decrease Activation Energy a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. A small quantity of catalyst should be able to. It does not lower the activation energy of the reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. catalysts increase. Why Catalyst Decrease Activation Energy.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free Why Catalyst Decrease Activation Energy That alternative route has a lower. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst provides an alternative route for the reaction with a lower activation energy. Catalysts are not consumed by the reaction. effect of enzymes and catalysts. A catalyst provides. Why Catalyst Decrease Activation Energy.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Why Catalyst Decrease Activation Energy It does not lower the activation energy of the reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. a catalyst is able to reduce the activation energy by forming a transition state in a. Why Catalyst Decrease Activation Energy.
From slideplayer.com
Chapter 24 Chemical Reactions and Enzymes. ppt download Why Catalyst Decrease Activation Energy in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. That alternative route has a lower. A catalyst provides an alternative route for the reaction. Enzymes are examples of catalysts. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines. Why Catalyst Decrease Activation Energy.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Why Catalyst Decrease Activation Energy a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. catalysts increase the rate of reaction. adding a catalyst has exactly this effect of shifting the activation energy. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. effect. Why Catalyst Decrease Activation Energy.
From ceupgijk.blob.core.windows.net
A Catalyst Lowers Activation Energy By at Maud Keen blog Why Catalyst Decrease Activation Energy in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. a catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst. Why Catalyst Decrease Activation Energy.
From ceenbrfk.blob.core.windows.net
Catalysts Lower The Activation Energy Required For A Reaction at Elnora Why Catalyst Decrease Activation Energy a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. Catalysts are not consumed by the reaction. A small quantity of catalyst should be able to. It does not lower the activation. Why Catalyst Decrease Activation Energy.
From ceupgijk.blob.core.windows.net
A Catalyst Lowers Activation Energy By at Maud Keen blog Why Catalyst Decrease Activation Energy in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. catalysts increase the rate of reaction. Enzymes are examples of catalysts. Catalysts are not consumed by. Why Catalyst Decrease Activation Energy.
From cefyhlmc.blob.core.windows.net
Why Are Enzymes Able To Lower Activation Energy at Linda Turner blog Why Catalyst Decrease Activation Energy adding a catalyst has exactly this effect of shifting the activation energy. a catalyst provides an alternative route for the reaction with a lower activation energy. Enzymes are examples of catalysts. effect of enzymes and catalysts. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. . Why Catalyst Decrease Activation Energy.
From www.slideserve.com
PPT Enzyme … the Biological Catalyst PowerPoint Presentation, free Why Catalyst Decrease Activation Energy a catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst lowers the activation energy of a chemical reaction. Enzymes are examples of catalysts. A catalyst provides an alternative route for the reaction. Catalysts are not consumed by the reaction. a catalyst is able to reduce the activation energy by forming a transition. Why Catalyst Decrease Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Why Catalyst Decrease Activation Energy a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. adding a catalyst has exactly this effect of shifting the activation energy. That alternative route has a lower. A catalyst lowers the activation energy of a chemical reaction. Enzymes are examples of catalysts. in the case of a. Why Catalyst Decrease Activation Energy.
From byjus.com
Activation Energy Why Catalyst Decrease Activation Energy Catalysts are not consumed by the reaction. Enzymes are examples of catalysts. effect of enzymes and catalysts. a catalyst provides an alternative route for the reaction with a lower activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. That alternative route has. Why Catalyst Decrease Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Why Catalyst Decrease Activation Energy A catalyst lowers the activation energy of a chemical reaction. catalysts increase the rate of reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst provides an alternative route for the reaction with a lower activation energy. effect of enzymes and. Why Catalyst Decrease Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Why Catalyst Decrease Activation Energy a catalyst provides an alternative route for the reaction with a lower activation energy. effect of enzymes and catalysts. Enzymes are examples of catalysts. catalysts increase the rate of reaction. adding a catalyst has exactly this effect of shifting the activation energy. as can be seen from the arrhenius equation, the magnitude of the activation. Why Catalyst Decrease Activation Energy.
From slideplayer.com
A catalyst lowers activation energy. ppt download Why Catalyst Decrease Activation Energy It does not lower the activation energy of the reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. A small quantity of catalyst should be. Why Catalyst Decrease Activation Energy.
From cetylqdp.blob.core.windows.net
Describe How Enzymes Lower Activation Energy at Patricia Chambers blog Why Catalyst Decrease Activation Energy catalysts increase the rate of reaction. A small quantity of catalyst should be able to. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. That alternative route. Why Catalyst Decrease Activation Energy.
From www.savemyexams.com
Catalysis OCR A Level Chemistry Revision Notes 2017 Why Catalyst Decrease Activation Energy Enzymes are examples of catalysts. A catalyst lowers the activation energy of a chemical reaction. A small quantity of catalyst should be able to. a catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst provides an alternative route for the reaction. That alternative route has a lower. a catalyst is able to. Why Catalyst Decrease Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Why Catalyst Decrease Activation Energy a catalyst provides an alternative route for the reaction with a lower activation energy. Catalysts are not consumed by the reaction. That alternative route has a lower. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. catalysts increase the rate of reaction. A small quantity of catalyst. Why Catalyst Decrease Activation Energy.
From ceenbrfk.blob.core.windows.net
Catalysts Lower The Activation Energy Required For A Reaction at Elnora Why Catalyst Decrease Activation Energy effect of enzymes and catalysts. A small quantity of catalyst should be able to. A catalyst lowers the activation energy of a chemical reaction. It does not lower the activation energy of the reaction. catalysts increase the rate of reaction. A catalyst provides an alternative route for the reaction. Catalysts are not consumed by the reaction. a. Why Catalyst Decrease Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Why Catalyst Decrease Activation Energy catalysts increase the rate of reaction. adding a catalyst has exactly this effect of shifting the activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst. Why Catalyst Decrease Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Why Catalyst Decrease Activation Energy a catalyst provides an alternative route for the reaction with a lower activation energy. adding a catalyst has exactly this effect of shifting the activation energy. It does not lower the activation energy of the reaction. A catalyst lowers the activation energy of a chemical reaction. a catalyst is able to reduce the activation energy by forming. Why Catalyst Decrease Activation Energy.
From ceugvfxv.blob.core.windows.net
Catalyst Activation Energy Graph at Beard blog Why Catalyst Decrease Activation Energy effect of enzymes and catalysts. It does not lower the activation energy of the reaction. A small quantity of catalyst should be able to. Enzymes are examples of catalysts. adding a catalyst has exactly this effect of shifting the activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to. Why Catalyst Decrease Activation Energy.
From slideplayer.com
Section 24 & 25 “Chemical Reactions & Enzymes” ppt download Why Catalyst Decrease Activation Energy a catalyst provides an alternative route for the reaction with a lower activation energy. Enzymes are examples of catalysts. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. A catalyst lowers the activation energy of a chemical reaction. That alternative route has a lower. as can be. Why Catalyst Decrease Activation Energy.
From slideplayer.com
EQUILIBRIUM. ppt download Why Catalyst Decrease Activation Energy That alternative route has a lower. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. a catalyst provides an alternative route for the reaction with a lower activation energy. adding a catalyst has exactly this effect of shifting the activation energy. A catalyst lowers the activation energy. Why Catalyst Decrease Activation Energy.
From www.slideserve.com
PPT Enzymes (Part I) PowerPoint Presentation, free download ID2525494 Why Catalyst Decrease Activation Energy A small quantity of catalyst should be able to. A catalyst lowers the activation energy of a chemical reaction. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. catalysts increase the rate of reaction. adding a catalyst has exactly this effect of shifting the activation energy. That. Why Catalyst Decrease Activation Energy.
From www.energyfrontier.us
When Less Is More Energy Frontier Research Center Why Catalyst Decrease Activation Energy A catalyst lowers the activation energy of a chemical reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the. Enzymes are examples of catalysts. a catalyst is able to reduce the activation energy by forming. Why Catalyst Decrease Activation Energy.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical Why Catalyst Decrease Activation Energy A catalyst lowers the activation energy of a chemical reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. A small quantity of catalyst should be able to. It does not lower the activation energy of the reaction. Catalysts are not consumed by the reaction. That. Why Catalyst Decrease Activation Energy.
From circuitdiagramlows.z22.web.core.windows.net
Catalyst Energy Diagram Why Catalyst Decrease Activation Energy in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. adding a catalyst has exactly this effect of shifting the activation energy. It does not lower. Why Catalyst Decrease Activation Energy.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Why Catalyst Decrease Activation Energy a catalyst is able to reduce the activation energy by forming a transition state in a more favorable manner. A catalyst lowers the activation energy of a chemical reaction. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. It does not lower the activation energy. Why Catalyst Decrease Activation Energy.