Titration Curve Hcl . a titration curve is a plot of some solution property versus the amount of added titrant. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. The shape of the curve will be the same as when you had an. Compute sample ph at important stages of a titration. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. at the beginning of this titration, you have an excess of hydrochloric acid. a titration curve is a plot of some solution property versus the amount of added titrant. Determine the concentration of analyte present, as well as the acid ionization constant and.
from www.chegg.com
at the beginning of this titration, you have an excess of hydrochloric acid. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. a titration curve is a plot of some solution property versus the amount of added titrant. a titration curve is a plot of some solution property versus the amount of added titrant. Determine the concentration of analyte present, as well as the acid ionization constant and. The shape of the curve will be the same as when you had an. Compute sample ph at important stages of a titration. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the.
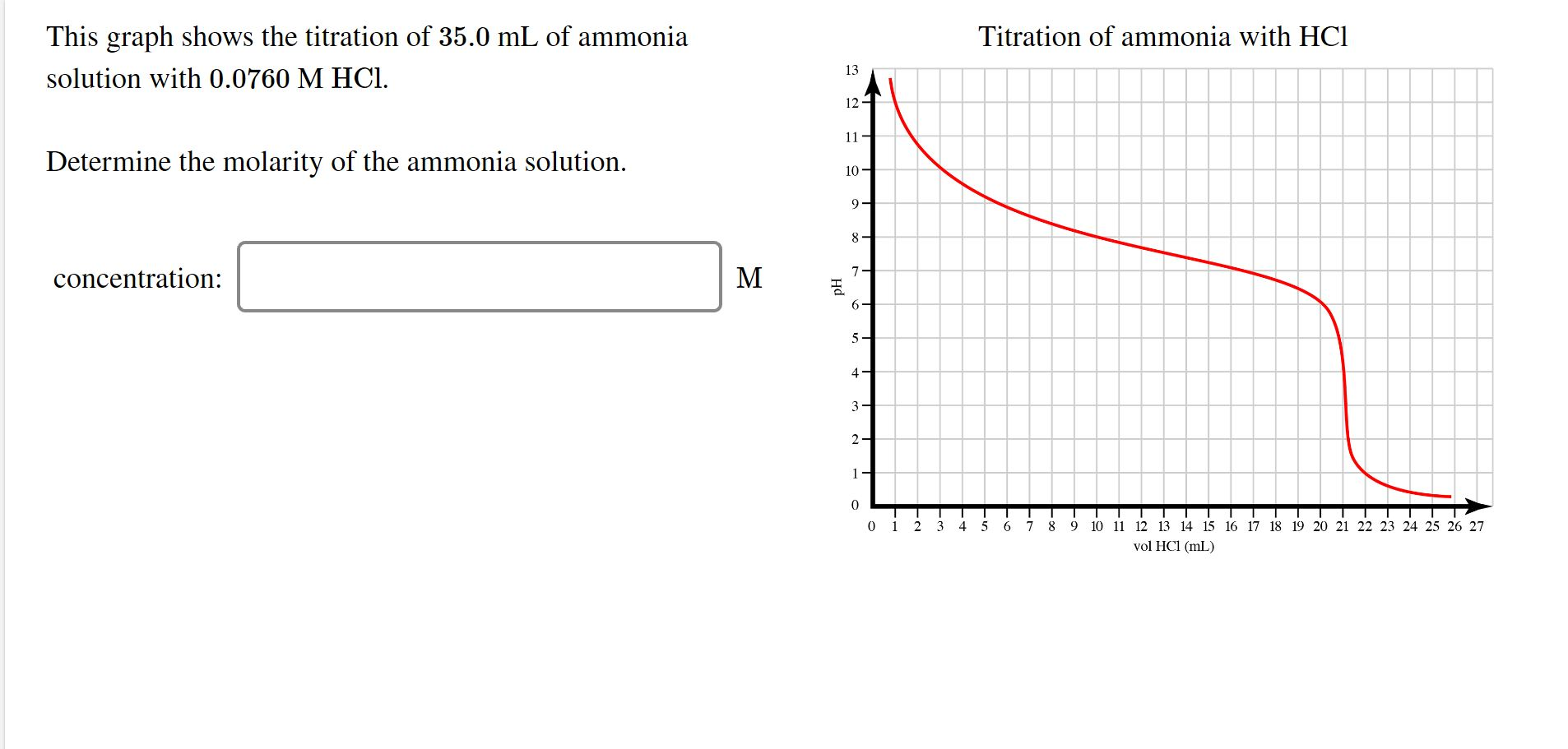
Solved Titration of ammonia with HCl This graph shows the
Titration Curve Hcl in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. Determine the concentration of analyte present, as well as the acid ionization constant and. The shape of the curve will be the same as when you had an. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. Compute sample ph at important stages of a titration. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. a titration curve is a plot of some solution property versus the amount of added titrant. a titration curve is a plot of some solution property versus the amount of added titrant. at the beginning of this titration, you have an excess of hydrochloric acid.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Titration Curve Hcl a titration curve is a plot of some solution property versus the amount of added titrant. at the beginning of this titration, you have an excess of hydrochloric acid. Determine the concentration of analyte present, as well as the acid ionization constant and. a titration curve is a plot of some solution property versus the amount of. Titration Curve Hcl.
From schoolbag.info
Titration and Buffers Acids and Bases Training MCAT General Titration Curve Hcl Determine the concentration of analyte present, as well as the acid ionization constant and. Compute sample ph at important stages of a titration. at the beginning of this titration, you have an excess of hydrochloric acid. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the.. Titration Curve Hcl.
From www.deborahsilvermusic.com
Solved HCl NaOH Titration Curve CH3COOH NaOH Titration, 47 OFF Titration Curve Hcl in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. a titration curve is a plot of some solution property versus the amount of added titrant.. Titration Curve Hcl.
From mungfali.com
Titration Curve Graph Titration Curve Hcl Compute sample ph at important stages of a titration. a titration curve is a plot of some solution property versus the amount of added titrant. at the beginning of this titration, you have an excess of hydrochloric acid. Determine the concentration of analyte present, as well as the acid ionization constant and. in this section we will. Titration Curve Hcl.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Hcl in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. at the beginning of this titration, you have an excess of hydrochloric acid. a titration. Titration Curve Hcl.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID4340170 Titration Curve Hcl a titration curve is a plot of some solution property versus the amount of added titrant. The shape of the curve will be the same as when you had an. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. in this section we will. Titration Curve Hcl.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Titration Curve Hcl The shape of the curve will be the same as when you had an. at the beginning of this titration, you have an excess of hydrochloric acid. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. Determine the concentration of analyte present, as well as. Titration Curve Hcl.
From www.researchgate.net
(A) Titration curve with HCl 0.1 M of interstitial water sample. (B Titration Curve Hcl Compute sample ph at important stages of a titration. a titration curve is a plot of some solution property versus the amount of added titrant. The shape of the curve will be the same as when you had an. at the beginning of this titration, you have an excess of hydrochloric acid. Determine the concentration of analyte present,. Titration Curve Hcl.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Curve Hcl in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. The shape of the curve will be the same as when you had an. Determine the concentration of analyte present, as well as the acid ionization constant and. at the beginning of this titration, you have an excess of. Titration Curve Hcl.
From mungfali.com
HCl NaOH Titration Titration Curve Hcl a titration curve is a plot of some solution property versus the amount of added titrant. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. The shape of the curve will be the same as when you had an. in an indicator based titration you add another. Titration Curve Hcl.
From www.chegg.com
Solved Given the titration curve for a titration between Titration Curve Hcl at the beginning of this titration, you have an excess of hydrochloric acid. a titration curve is a plot of some solution property versus the amount of added titrant. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. The shape of the curve will. Titration Curve Hcl.
From www.researchgate.net
Simulated titration curve of 3 mL each of 0.1 M HCl (strong acid), 0.1 Titration Curve Hcl in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. a titration curve is a plot of some solution property versus the amount of added titrant. Compute sample ph at important stages of a titration. a titration curve is a plot of some solution property. Titration Curve Hcl.
From fity.club
Titration Of Hcl With Naoh Titration Curve Hcl in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. a titration curve is a plot of some solution property versus the amount of added titrant. a titration curve is a plot of some solution property versus the amount of added titrant. The shape of the curve will. Titration Curve Hcl.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Hcl Determine the concentration of analyte present, as well as the acid ionization constant and. at the beginning of this titration, you have an excess of hydrochloric acid. Compute sample ph at important stages of a titration. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the.. Titration Curve Hcl.
From www.slideserve.com
PPT Acids Lesson 18 Titration Curves PowerPoint Presentation, free Titration Curve Hcl Compute sample ph at important stages of a titration. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. at the beginning of this titration, you. Titration Curve Hcl.
From www.youtube.com
Trimethylamine Titrated with HCl YouTube Titration Curve Hcl a titration curve is a plot of some solution property versus the amount of added titrant. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. Determine the concentration of analyte present, as well as the acid ionization constant and. a titration curve is a plot of some. Titration Curve Hcl.
From chart-studio.plotly.com
Titration Curve HCLH3PO4 with NaOH scatter chart made by Meerika Titration Curve Hcl a titration curve is a plot of some solution property versus the amount of added titrant. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6.. Titration Curve Hcl.
From mungfali.com
Titration Graph Titration Curve Hcl The shape of the curve will be the same as when you had an. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. at the. Titration Curve Hcl.
From exoqledql.blob.core.windows.net
Titration Base Hydrochloric Acid at Bryan Barnes blog Titration Curve Hcl in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. at the beginning of this titration, you have an excess of hydrochloric acid. a titration curve is a plot of some solution property versus the amount of added titrant. Determine the concentration of analyte present, as well as. Titration Curve Hcl.
From fity.club
Titration Of Hcl With Naoh Titration Curve Hcl Compute sample ph at important stages of a titration. at the beginning of this titration, you have an excess of hydrochloric acid. The shape of the curve will be the same as when you had an. Determine the concentration of analyte present, as well as the acid ionization constant and. in this section we will learn how to. Titration Curve Hcl.
From mungfali.com
Titration Curve HCl And NaOH Titration Curve Hcl in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. Compute sample ph at important stages of a titration. at the beginning of this titration, you have an excess of hydrochloric acid. a titration curve is a plot of some solution property versus the amount. Titration Curve Hcl.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong Titration Curve Hcl Compute sample ph at important stages of a titration. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. Determine the concentration of analyte present, as well as the acid ionization constant and. The shape of the curve will be the same as when you had an. a titration. Titration Curve Hcl.
From www.learnsci.com
LearnSci Smart Worksheet Determine Concentration of HCl by Titration Titration Curve Hcl a titration curve is a plot of some solution property versus the amount of added titrant. a titration curve is a plot of some solution property versus the amount of added titrant. Compute sample ph at important stages of a titration. in this section we will learn how to calculate a titration curve using the equilibrium calculations. Titration Curve Hcl.
From mungfali.com
Titration Curve HCl And NaOH Titration Curve Hcl in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. at the beginning of this titration, you have an excess of hydrochloric acid. The shape of the curve will be the same as when you had an. a titration curve is a plot of some solution property versus. Titration Curve Hcl.
From www.sarthaks.com
Predict titration curve for titration of `Na(2)CO_(3)` & `HCl Titration Curve Hcl Determine the concentration of analyte present, as well as the acid ionization constant and. at the beginning of this titration, you have an excess of hydrochloric acid. Compute sample ph at important stages of a titration. The shape of the curve will be the same as when you had an. in an indicator based titration you add another. Titration Curve Hcl.
From mavink.com
Hcl Titration Curve Titration Curve Hcl Compute sample ph at important stages of a titration. a titration curve is a plot of some solution property versus the amount of added titrant. a titration curve is a plot of some solution property versus the amount of added titrant. at the beginning of this titration, you have an excess of hydrochloric acid. in this. Titration Curve Hcl.
From www.researchgate.net
Titration curve of HEPESsol, HEPES solution in HCl acidic medium by Titration Curve Hcl a titration curve is a plot of some solution property versus the amount of added titrant. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. a titration curve is a plot of some solution property versus the amount of added titrant. in this. Titration Curve Hcl.
From chart-studio.plotly.com
Titration Curve of Glycine HCl scatter chart made by Cvaltier plotly Titration Curve Hcl Determine the concentration of analyte present, as well as the acid ionization constant and. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. The shape of the curve will be the same as when you had an. a titration curve is a plot of some. Titration Curve Hcl.
From www.deborahsilvermusic.com
Titration Of HCL With Naoh Milly's Portfolio, 40 OFF Titration Curve Hcl at the beginning of this titration, you have an excess of hydrochloric acid. a titration curve is a plot of some solution property versus the amount of added titrant. The shape of the curve will be the same as when you had an. in an indicator based titration you add another chemical that changes color at the. Titration Curve Hcl.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration Curve Hcl in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. a titration curve is a plot of some solution property versus the amount of added titrant. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6.. Titration Curve Hcl.
From mungfali.com
HCl NaOH Titration Titration Curve Hcl Compute sample ph at important stages of a titration. a titration curve is a plot of some solution property versus the amount of added titrant. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. a titration curve is a plot of some solution property. Titration Curve Hcl.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration Curve Hcl The shape of the curve will be the same as when you had an. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. a titration curve is a plot of some solution property versus the amount of added titrant. in this section we will. Titration Curve Hcl.
From fity.club
Titration Of Hcl With Naoh Titration Curve Hcl a titration curve is a plot of some solution property versus the amount of added titrant. in this section we will learn how to calculate a titration curve using the equilibrium calculations from chapter 6. The shape of the curve will be the same as when you had an. at the beginning of this titration, you have. Titration Curve Hcl.
From www.chegg.com
Solved Titration of ammonia with HCl This graph shows the Titration Curve Hcl The shape of the curve will be the same as when you had an. Determine the concentration of analyte present, as well as the acid ionization constant and. at the beginning of this titration, you have an excess of hydrochloric acid. a titration curve is a plot of some solution property versus the amount of added titrant. . Titration Curve Hcl.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve Hcl The shape of the curve will be the same as when you had an. Compute sample ph at important stages of a titration. a titration curve is a plot of some solution property versus the amount of added titrant. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence. Titration Curve Hcl.