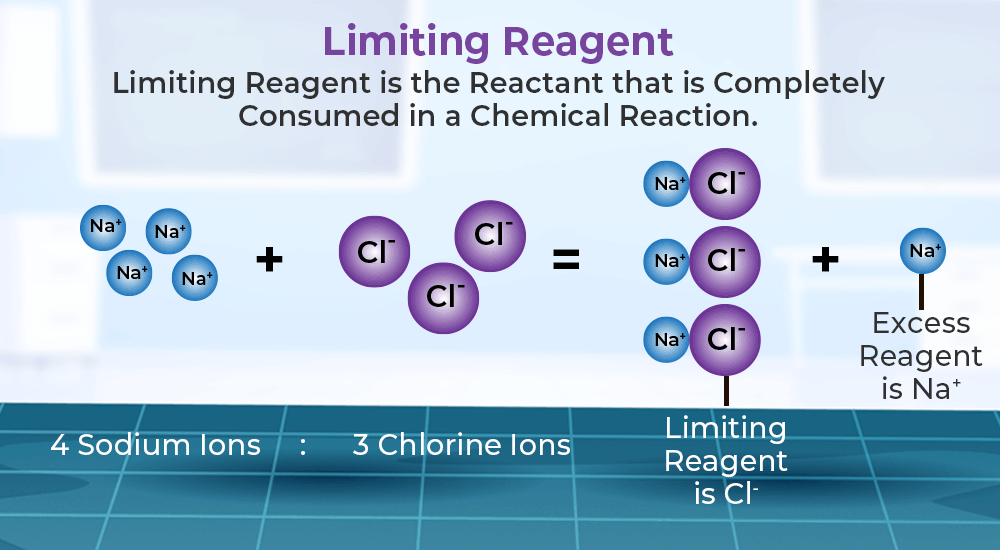
Ice Table Limiting Reactant . using ice charts to do limiting reactant problems limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. We therefore put a zero under hydrogen in the end row of the ice table. Convert any amounts you are given. Write a balanced chemical equation describing the reaction in the problem. an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. Determine which of two reactants is the limiting reactant. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. Find the limiting reactant and set its value at the end to zero in the table. The column for hydrogen gives a simple equation that. We have only 4 mol, hence hydrogen is the limiting reactant.
from www.geeksforgeeks.org
Write a balanced chemical equation describing the reaction in the problem. The column for hydrogen gives a simple equation that. Convert any amounts you are given. an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. Determine which of two reactants is the limiting reactant. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. using ice charts to do limiting reactant problems We therefore put a zero under hydrogen in the end row of the ice table. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen.
Limiting Reagent Definition, Methods, Solved Examples, and FAQs
Ice Table Limiting Reactant Convert any amounts you are given. Write a balanced chemical equation describing the reaction in the problem. an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. We therefore put a zero under hydrogen in the end row of the ice table. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. using ice charts to do limiting reactant problems The column for hydrogen gives a simple equation that. Find the limiting reactant and set its value at the end to zero in the table. We have only 4 mol, hence hydrogen is the limiting reactant. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. Convert any amounts you are given. Determine which of two reactants is the limiting reactant.
From www.youtube.com
Excess and Limiting w ICE Tables Practice 2 YouTube Ice Table Limiting Reactant Write a balanced chemical equation describing the reaction in the problem. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. using ice charts to do limiting reactant problems We therefore put a zero under hydrogen in the end row of the ice table. Convert any amounts you are given. an ice. Ice Table Limiting Reactant.
From www.geeksforgeeks.org
Limiting Reagent Definition, Methods, Solved Examples, and FAQs Ice Table Limiting Reactant Determine which of two reactants is the limiting reactant. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. Write a balanced chemical equation describing the reaction in the problem. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. Find the limiting reactant and set its value. Ice Table Limiting Reactant.
From www.studocu.com
Outline 2 Equilibrium Concentrations using ICE Tables, Reaction Ice Table Limiting Reactant limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. Determine which of two. Ice Table Limiting Reactant.
From quizzlistoutmantle.z21.web.core.windows.net
How To Calculate Limiting And Excess Reactant Ice Table Limiting Reactant Determine which of two reactants is the limiting reactant. Write a balanced chemical equation describing the reaction in the problem. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. using ice charts to do limiting reactant problems We have only 4 mol, hence hydrogen is the limiting reactant. Find the limiting reactant. Ice Table Limiting Reactant.
From www.youtube.com
Using ICE Tables to find Eq. Concentrations & Kc YouTube Ice Table Limiting Reactant Determine which of two reactants is the limiting reactant. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. We therefore put a zero under hydrogen in the end row of the ice table. Find the limiting reactant and set its value at the end to zero in the table. using ice charts. Ice Table Limiting Reactant.
From www.slideserve.com
PPT Theoretical Yield Which Reactant is Limiting? PowerPoint Ice Table Limiting Reactant The column for hydrogen gives a simple equation that. Find the limiting reactant and set its value at the end to zero in the table. Determine which of two reactants is the limiting reactant. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. Convert any amounts you are given. using ice charts. Ice Table Limiting Reactant.
From studyoranjehairse.z13.web.core.windows.net
How To Find Limiting Reagent Stoichiometry Ice Table Limiting Reactant Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. Determine which of two reactants is the limiting reactant. We have only 4 mol, hence hydrogen is the limiting reactant. an ice table. Ice Table Limiting Reactant.
From slidetodoc.com
ICE Tables and Equilibrium Concentrations Lesson Outline ICE Ice Table Limiting Reactant Convert any amounts you are given. We therefore put a zero under hydrogen in the end row of the ice table. using ice charts to do limiting reactant problems an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. Determine which of two reactants is the limiting reactant. Here,. Ice Table Limiting Reactant.
From chemistry.analia-sanchez.net
Limiting Reactant Notes Chemistry Classes / Ronald Reagan S.H.S. Ice Table Limiting Reactant the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. Find the limiting reactant and set its value at the end to zero in the table. Determine which of two reactants is the limiting reactant. Convert any amounts you are given. We have only 4 mol, hence hydrogen is the. Ice Table Limiting Reactant.
From www.youtube.com
Quadratic Equation ICE Table Equilibrium Calculations YouTube Ice Table Limiting Reactant Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. Find the limiting reactant and set its value at the end to zero in the table. The column for hydrogen gives a simple equation that. We therefore put a zero under hydrogen in the end row of the ice table. limiting reagents using. Ice Table Limiting Reactant.
From www.youtube.com
Limiting Reactants and BCA Tables YouTube Ice Table Limiting Reactant Convert any amounts you are given. Determine which of two reactants is the limiting reactant. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. Write a balanced chemical equation describing the reaction in the problem. Find the limiting reactant and set its value at the end to zero in the table. We have. Ice Table Limiting Reactant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Ice Table Limiting Reactant using ice charts to do limiting reactant problems The column for hydrogen gives a simple equation that. We have only 4 mol, hence hydrogen is the limiting reactant. an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. the x value can be used to calculate the equilibrium. Ice Table Limiting Reactant.
From studyhypnotised.z21.web.core.windows.net
When Do You Use Ice Tables Ice Table Limiting Reactant an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. Convert any amounts you are given. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. Find the limiting reactant and set its value at the end to zero in the table. limiting. Ice Table Limiting Reactant.
From www.numerade.com
SOLVED 8. Complete an ICE table for 1.0 mol SO3 reacting with 2.0 mol Ice Table Limiting Reactant an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. The column for hydrogen gives a simple equation that. Find the limiting reactant and set its value at the end. Ice Table Limiting Reactant.
From www.youtube.com
ICE Tables Given K but NO Equilibrium Concentrations YouTube Ice Table Limiting Reactant an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. Determine which of two reactants is the limiting reactant. The column for hydrogen gives a simple equation that. We have only 4 mol, hence hydrogen is the limiting reactant. limiting reagents using ice tables here, we demonstrate quantitative calculations. Ice Table Limiting Reactant.
From www.youtube.com
ICE Chart (Limiting Reactants) Example 1 YouTube Ice Table Limiting Reactant Find the limiting reactant and set its value at the end to zero in the table. an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. the x value can be used to. Ice Table Limiting Reactant.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Ice Table Limiting Reactant the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. Determine which of two reactants is the limiting reactant. We therefore put a zero under hydrogen in the end row of the ice table. Convert any amounts you are given. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63. Ice Table Limiting Reactant.
From www.youtube.com
completing table for limiting reactants YouTube Ice Table Limiting Reactant an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. Convert any amounts you are given. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. Determine which of two reactants is the limiting reactant. using ice charts to do limiting reactant problems. Ice Table Limiting Reactant.
From www.youtube.com
CHEM 101 Solving Limiting Reagent Problems using a Limiting Reagent Ice Table Limiting Reactant using ice charts to do limiting reactant problems Write a balanced chemical equation describing the reaction in the problem. We therefore put a zero under hydrogen in the end row of the ice table. an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. Convert any amounts you are. Ice Table Limiting Reactant.
From www.youtube.com
Limiting Reactant Stoichiometry using ICE Tables YouTube Ice Table Limiting Reactant limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. using ice charts to do limiting reactant problems Find the limiting reactant and set its value at the end to zero in the table. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging. Ice Table Limiting Reactant.
From chemistry.analia-sanchez.net
Limiting Reactant Notes Chemistry Classes / Ronald Reagan S.H.S. Ice Table Limiting Reactant We therefore put a zero under hydrogen in the end row of the ice table. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. using ice charts to do limiting reactant problems Find the limiting reactant and set its value at the end to zero in the table. Determine which of two. Ice Table Limiting Reactant.
From www.youtube.com
Equilibrium 5.4 ICE Tables YouTube Ice Table Limiting Reactant the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. We have only 4 mol, hence hydrogen is the limiting reactant. an ice table is a tool used to calculate the changing concentrations. Ice Table Limiting Reactant.
From www.youtube.com
Using ICE Tables (aka RICE Chart) in Equilibrium Calculations with Ice Table Limiting Reactant We have only 4 mol, hence hydrogen is the limiting reactant. Find the limiting reactant and set its value at the end to zero in the table. Determine which of two reactants is the limiting reactant. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. We therefore put a zero under hydrogen in. Ice Table Limiting Reactant.
From www.youtube.com
Chemical Equilibrium V The ICE Table Introduction YouTube Ice Table Limiting Reactant Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of hydrogen. Write a balanced chemical equation describing the reaction in the problem. We have only 4 mol, hence hydrogen is the limiting reactant. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. We therefore put a zero under. Ice Table Limiting Reactant.
From www.numerade.com
SOLVED The balanced equation for the reaction of nitrogen and fluorine Ice Table Limiting Reactant limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. We therefore put a zero under hydrogen in the end row of the ice table. Find the limiting reactant and set its value at. Ice Table Limiting Reactant.
From www.youtube.com
Limiting reagent ICE diagram YouTube Ice Table Limiting Reactant limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. using ice charts to do limiting reactant problems Find the limiting reactant and set its value at the end to zero in the table. Write a balanced chemical equation describing the reaction in the problem. We have only 4 mol, hence hydrogen is. Ice Table Limiting Reactant.
From www.youtube.com
Chem Unit 8 Limiting Reactant with BCA YouTube Ice Table Limiting Reactant limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. Find the limiting reactant and set its value at the end to zero in the table. using ice charts to do limiting reactant problems the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging. Ice Table Limiting Reactant.
From www.youtube.com
Excess and Limiting Reactants Using ICE Tables YouTube Ice Table Limiting Reactant the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. Find the limiting reactant and set its value at the end to zero in the table. The column for hydrogen gives a simple equation that. using ice charts to do limiting reactant problems Determine which of two reactants is. Ice Table Limiting Reactant.
From z-cm.blogspot.com
Ice Table Chem Decoration Examples Ice Table Limiting Reactant the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. Determine which of two reactants is the limiting reactant. Find the limiting reactant and set its value at the end to zero in the table. Here, 1.63 mol of iron(iii) oxide requires 3 x 1.63 mol = 4.89 mol of. Ice Table Limiting Reactant.
From www.slideserve.com
PPT Limiting Reactants and ICE Charts PowerPoint Presentation, free Ice Table Limiting Reactant an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. Find the limiting reactant and set its value at the end to zero in the table. Convert any amounts you are given. Write a balanced chemical equation describing the reaction in the problem. using ice charts to do limiting. Ice Table Limiting Reactant.
From chemistry.analia-sanchez.net
Limiting Reactant Notes Chemistry Classes / Ronald Reagan S.H.S. Ice Table Limiting Reactant We therefore put a zero under hydrogen in the end row of the ice table. Determine which of two reactants is the limiting reactant. limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it.. Ice Table Limiting Reactant.
From www.scienceabc.com
How To Find The Limiting Reactant In A Chemical Reaction? Ice Table Limiting Reactant the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. Write a balanced chemical equation describing the reaction in the problem. We therefore put a zero under hydrogen in the end row of the ice table. Convert any amounts you are given. Find the limiting reactant and set its value. Ice Table Limiting Reactant.
From www.youtube.com
Reaction quotients and ICE tables YouTube Ice Table Limiting Reactant an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it. Write a balanced chemical equation describing the reaction in the problem. We therefore put a zero under hydrogen in the. Ice Table Limiting Reactant.
From www.slideserve.com
PPT Limiting Reactants and ICE Charts PowerPoint Presentation, free Ice Table Limiting Reactant We have only 4 mol, hence hydrogen is the limiting reactant. Convert any amounts you are given. Determine which of two reactants is the limiting reactant. using ice charts to do limiting reactant problems limiting reagents using ice tables here, we demonstrate quantitative calculations to determine final concentrations in. Write a balanced chemical equation describing the reaction in. Ice Table Limiting Reactant.
From www.youtube.com
Stoichiometry Limiting Reagent (ICE Box) YouTube Ice Table Limiting Reactant Convert any amounts you are given. using ice charts to do limiting reactant problems Find the limiting reactant and set its value at the end to zero in the table. We have only 4 mol, hence hydrogen is the limiting reactant. Write a balanced chemical equation describing the reaction in the problem. The column for hydrogen gives a simple. Ice Table Limiting Reactant.