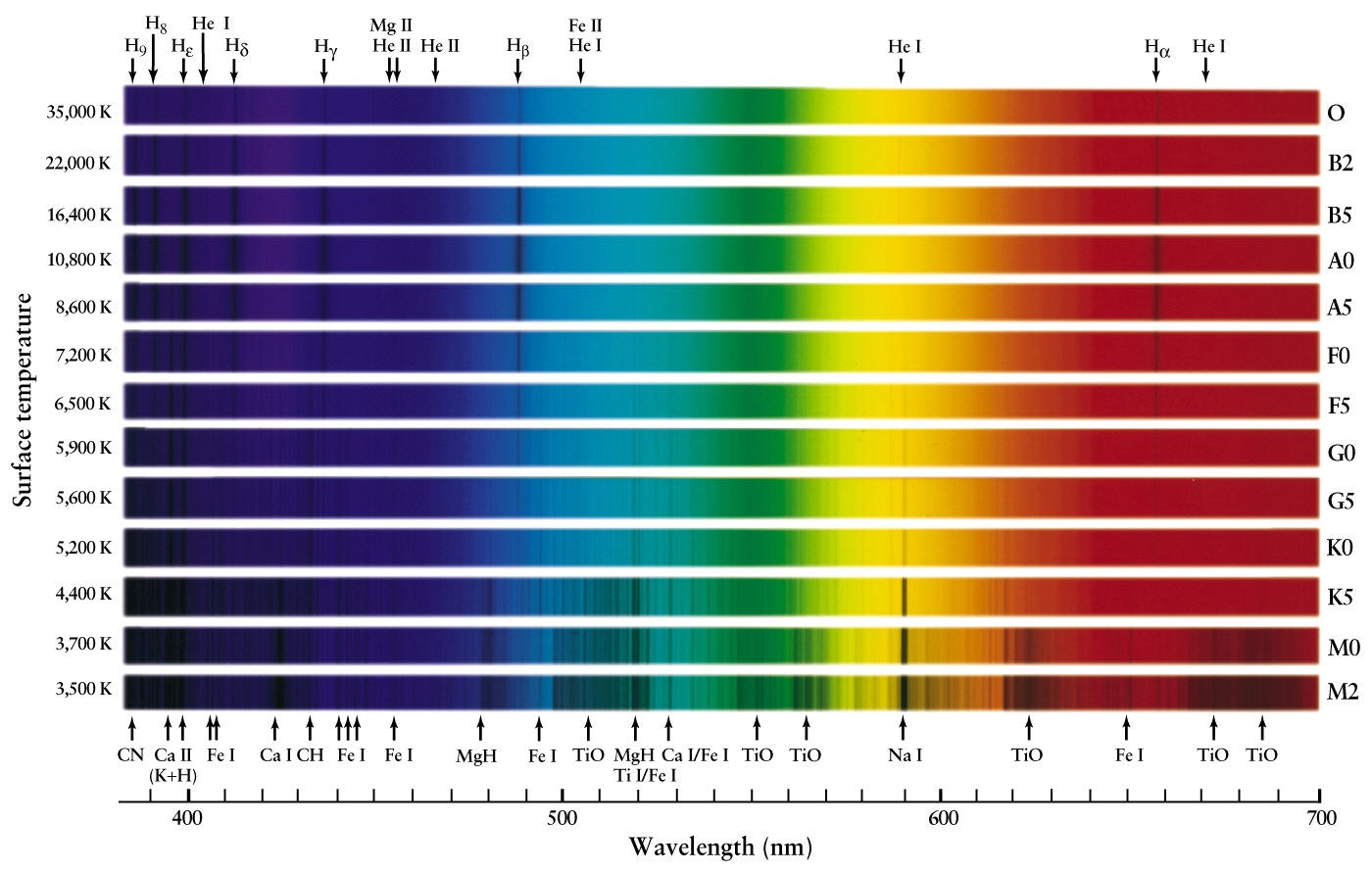
What Are Bright-Line Spectrum Used For . If you have ever let food boil over when cooking, you will notice the gas flame will turn a. the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. You will be able to distinguish between emission and absorption lines in a spectrum. You will know how spectral lines are. the line spectrum is like a fingerprint and is specific for each element. a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1.
from www.howtuo.com
a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. You will know how spectral lines are. this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. the line spectrum is like a fingerprint and is specific for each element. the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). If you have ever let food boil over when cooking, you will notice the gas flame will turn a. You will be able to distinguish between emission and absorption lines in a spectrum. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on.
The Bright Lines Of An Emission Spectrum Are The Result Of • HOWTUO
What Are Bright-Line Spectrum Used For the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. the line spectrum is like a fingerprint and is specific for each element. You will know how spectral lines are. this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. You will be able to distinguish between emission and absorption lines in a spectrum. the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. If you have ever let food boil over when cooking, you will notice the gas flame will turn a. a hot gas only emits certain wavelengths of light to produce bright lines on a dark background.
From www.differencebetween.com
What is the Difference Between a Continuous Spectrum and a Bright Line What Are Bright-Line Spectrum Used For hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. You will know how spectral lines are. a hot gas only emits certain wavelengths of light. What Are Bright-Line Spectrum Used For.
From www.homedit.com
Color Spectrum The Meaning of Colors and How to Use Them What Are Bright-Line Spectrum Used For the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. You will be able to distinguish between emission and absorption lines in a spectrum. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. the line spectrum is like. What Are Bright-Line Spectrum Used For.
From fphoto.photoshelter.com
science physics waves bright line spectrum analysis What Are Bright-Line Spectrum Used For this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. If you have ever let food boil over when cooking, you will notice the gas flame will turn a.. What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT Chapter 4 Electron Configurations and Quantum Chemistry What Are Bright-Line Spectrum Used For You will be able to distinguish between emission and absorption lines in a spectrum. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. the line spectrum is like. What Are Bright-Line Spectrum Used For.
From www.transformationtutoring.com
Given the brightline spectra of four elements and the spectrum of a What Are Bright-Line Spectrum Used For If you have ever let food boil over when cooking, you will notice the gas flame will turn a. You will be able to distinguish between emission and absorption lines in a spectrum. the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. the light from an astronomical source. What Are Bright-Line Spectrum Used For.
From slideplayer.com
Reference Table Table S & PT ppt download What Are Bright-Line Spectrum Used For the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). If you have ever let food boil over when cooking, you will notice the gas flame will turn a. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured. What Are Bright-Line Spectrum Used For.
From www.aiophotoz.com
The Spectrum Images and Photos finder What Are Bright-Line Spectrum Used For hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). If you have ever let food boil over when cooking, you will notice the gas flame will. What Are Bright-Line Spectrum Used For.
From www.studocu.com
Bright LINE Spectra LAB Sheet BRIGHT LINE SPECTRA LAB Purpose The What Are Bright-Line Spectrum Used For If you have ever let food boil over when cooking, you will notice the gas flame will turn a. You will be able to distinguish between emission and absorption lines in a spectrum. the line spectrum is like a fingerprint and is specific for each element. the light from an astronomical source can consist of a continuous spectrum,. What Are Bright-Line Spectrum Used For.
From fphoto.photoshelter.com
science physics waves bright line spectrum analysis What Are Bright-Line Spectrum Used For this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. You will be able to distinguish between emission and absorption lines in a spectrum. the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line).. What Are Bright-Line Spectrum Used For.
From www.sliderbase.com
Electrons and WaveParticle Duality Presentation Chemistry What Are Bright-Line Spectrum Used For the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). You will know how spectral lines are. the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. If you have ever let food boil over when cooking,. What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT Electrons and Periodicity PowerPoint Presentation, free download What Are Bright-Line Spectrum Used For the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. If you have ever let food boil over when cooking, you will notice the gas flame will. What Are Bright-Line Spectrum Used For.
From quizlooyenwork.z21.web.core.windows.net
What Is Bright Line Spectrum What Are Bright-Line Spectrum Used For a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. the line spectrum is like a fingerprint and is specific for each element. You will know how spectral lines are. You. What Are Bright-Line Spectrum Used For.
From slideplayer.com
Atomic Structure. ppt download What Are Bright-Line Spectrum Used For this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. the line spectrum is like a fingerprint and is specific for each element. You will know how spectral lines are. the light from an astronomical source can consist of a continuous spectrum, an emission (bright. What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT Electrons PowerPoint Presentation, free download ID9601319 What Are Bright-Line Spectrum Used For a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. the line spectrum is like a fingerprint and is specific for each element. If you have ever let food boil over. What Are Bright-Line Spectrum Used For.
From www.youtube.com
Bright Line Spectra and Electrons YouTube What Are Bright-Line Spectrum Used For the line spectrum is like a fingerprint and is specific for each element. You will know how spectral lines are. a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. . What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT Chapter 4 Electron Configurations and Quantum Chemistry What Are Bright-Line Spectrum Used For a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. You will be able to distinguish between emission and absorption lines in a spectrum. the line spectrum is like a fingerprint. What Are Bright-Line Spectrum Used For.
From www.transformationtutoring.com
The brightline spectra of four elements, G, J, L, and M, and a mixture What Are Bright-Line Spectrum Used For If you have ever let food boil over when cooking, you will notice the gas flame will turn a. the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. You will be able to distinguish between emission and absorption lines in a spectrum. this produces an absorption spectrum, which. What Are Bright-Line Spectrum Used For.
From slideplayer.com
Atomic Structure. ppt download What Are Bright-Line Spectrum Used For If you have ever let food boil over when cooking, you will notice the gas flame will turn a. a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. You will be able to distinguish between emission and absorption lines in a spectrum. hence only certain frequencies of light are observed,. What Are Bright-Line Spectrum Used For.
From exoszwsiw.blob.core.windows.net
Emission Spectra Stars at Michael Ibarra blog What Are Bright-Line Spectrum Used For You will be able to distinguish between emission and absorption lines in a spectrum. If you have ever let food boil over when cooking, you will notice the gas flame will turn a. this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. the line spectrum. What Are Bright-Line Spectrum Used For.
From fphoto.photoshelter.com
science physics waves bright line spectrum analysis What Are Bright-Line Spectrum Used For If you have ever let food boil over when cooking, you will notice the gas flame will turn a. the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in. What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT Spectrum PowerPoint Presentation, free download What Are Bright-Line Spectrum Used For the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). You will know how spectral lines are. If you have ever let food boil over when cooking, you will notice the gas flame will turn a. the spectrum formed is an emission or bright line spectrum,. What Are Bright-Line Spectrum Used For.
From slideplayer.com
Modern Atomic Model. ppt download What Are Bright-Line Spectrum Used For If you have ever let food boil over when cooking, you will notice the gas flame will turn a. You will know how spectral lines are. a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. You will be able to distinguish between emission and absorption lines in a spectrum. hence. What Are Bright-Line Spectrum Used For.
From www.astronomynotes.com
Radiation What Are Bright-Line Spectrum Used For You will know how spectral lines are. You will be able to distinguish between emission and absorption lines in a spectrum. a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption. What Are Bright-Line Spectrum Used For.
From fphoto.photoshelter.com
science physics waves bright line spectrum analysis What Are Bright-Line Spectrum Used For the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). If you have ever let food boil over when cooking, you will notice the gas flame will turn a. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured. What Are Bright-Line Spectrum Used For.
From slideplayer.com
Atomic Structure. ppt download What Are Bright-Line Spectrum Used For the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. a hot gas only emits certain wavelengths of light to produce bright lines on a dark. What Are Bright-Line Spectrum Used For.
From www.howtuo.com
The Bright Lines Of An Emission Spectrum Are The Result Of • HOWTUO What Are Bright-Line Spectrum Used For the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. You will be able to distinguish between emission and absorption lines in a spectrum. hence only certain frequencies. What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT Light and the Spectrum PowerPoint Presentation What Are Bright-Line Spectrum Used For You will be able to distinguish between emission and absorption lines in a spectrum. If you have ever let food boil over when cooking, you will notice the gas flame will turn a. the light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line). the spectrum formed. What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT THE ATOM PowerPoint Presentation, free download ID6447001 What Are Bright-Line Spectrum Used For a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. You will know how spectral lines are. this produces an absorption spectrum, which has dark lines in the same position as. What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT EMISSION SPECTRUM PowerPoint Presentation ID5777174 What Are Bright-Line Spectrum Used For If you have ever let food boil over when cooking, you will notice the gas flame will turn a. You will know how spectral lines are. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. this produces an absorption spectrum, which has dark lines in the same position. What Are Bright-Line Spectrum Used For.
From www.mrpalermo.com
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom What Are Bright-Line Spectrum Used For a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. the line spectrum is like a fingerprint and is specific for each element. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. You will be able to distinguish between emission. What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT The Big Bang Theory PowerPoint Presentation, free download ID What Are Bright-Line Spectrum Used For the line spectrum is like a fingerprint and is specific for each element. hence only certain frequencies of light are observed, forming the emission spectrum, which is discrete bright coloured lines on. You will be able to distinguish between emission and absorption lines in a spectrum. If you have ever let food boil over when cooking, you will. What Are Bright-Line Spectrum Used For.
From fphoto.photoshelter.com
science physics waves spectrum analysis Fundamental What Are Bright-Line Spectrum Used For If you have ever let food boil over when cooking, you will notice the gas flame will turn a. You will know how spectral lines are. a hot gas only emits certain wavelengths of light to produce bright lines on a dark background. the line spectrum is like a fingerprint and is specific for each element. the. What Are Bright-Line Spectrum Used For.
From slideplayer.com
The Spectroscope New Meanings in Light ppt download What Are Bright-Line Spectrum Used For If you have ever let food boil over when cooking, you will notice the gas flame will turn a. the spectrum formed is an emission or bright line spectrum, as shown by the middle spectrum in figure 1. this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission. What Are Bright-Line Spectrum Used For.
From www.slideserve.com
PPT Electrons! PowerPoint Presentation, free download ID3909025 What Are Bright-Line Spectrum Used For this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. You will be able to distinguish between emission and absorption lines in a spectrum. the line spectrum is like a fingerprint and is specific for each element. If you have ever let food boil over when. What Are Bright-Line Spectrum Used For.
From www.transformationtutoring.com
The brightline spectra produced by four elements are represented in What Are Bright-Line Spectrum Used For the line spectrum is like a fingerprint and is specific for each element. If you have ever let food boil over when cooking, you will notice the gas flame will turn a. this produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of. a hot gas. What Are Bright-Line Spectrum Used For.