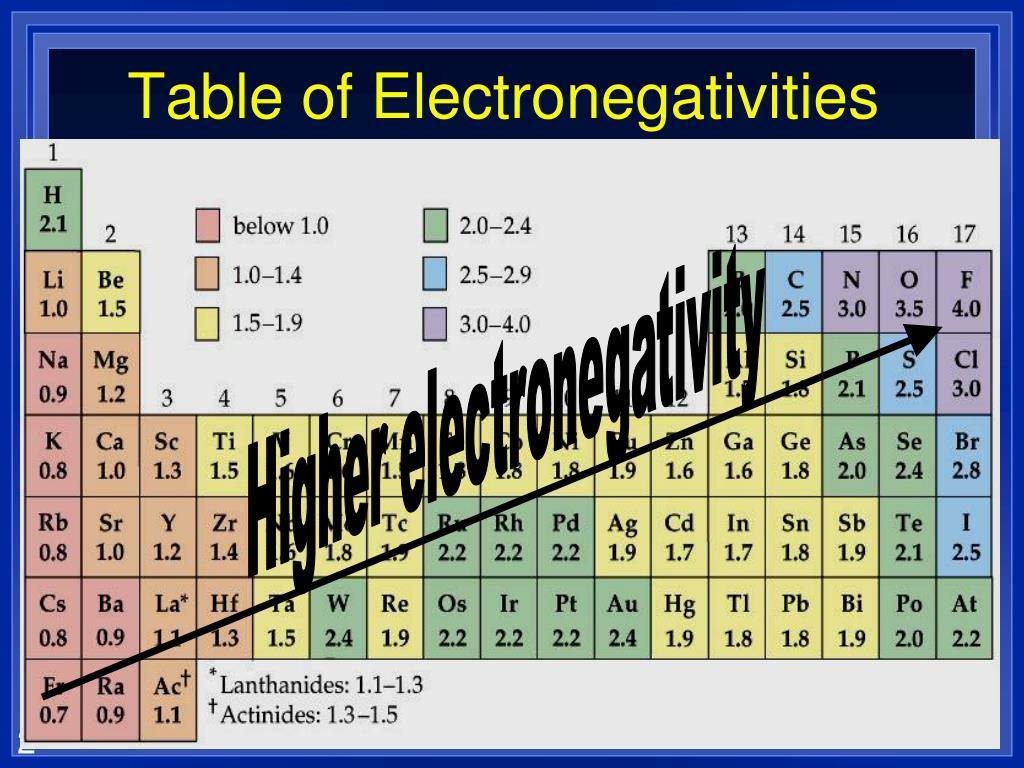
Element With Highest Electronegativity . 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. The suggested values are all taken from. fluorine is the most electronegative element. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. electronegativity is the tendency of an atom to attract electrons in a bond. Find out which element has. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. electronegativity is not a uniquely defined property and may depend on the definition. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Learn how to find electronegativity values, how they relate to bond.
from www.slideserve.com
119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. Find out which element has. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. electronegativity is not a uniquely defined property and may depend on the definition. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. electronegativity is the tendency of an atom to attract electrons in a bond. The suggested values are all taken from. fluorine is the most electronegative element. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound.
PPT Electronegativity? PowerPoint Presentation, free download ID
Element With Highest Electronegativity learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. electronegativity is the tendency of an atom to attract electrons in a bond. Find out which element has. 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. The suggested values are all taken from. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. electronegativity is not a uniquely defined property and may depend on the definition. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Learn how to find electronegativity values, how they relate to bond. fluorine is the most electronegative element.
From ar.inspiredpencil.com
Periodic Table With Electronegativity And Electron Configuration Element With Highest Electronegativity 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. Find out which element has. electronegativity is not a uniquely defined property and may depend on the definition. fluorine is the most electronegative element.. Element With Highest Electronegativity.
From www.geeksforgeeks.org
Electronegativity Definition, Meaning, Periodic Trends, Examples Element With Highest Electronegativity 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. electronegativity is the tendency of an atom to attract electrons in a bond. learn how to define and measure electronegativity, the ability of an. Element With Highest Electronegativity.
From www.vrogue.co
11 2 Electronegativity Chemistry Libretexts vrogue.co Element With Highest Electronegativity fluorine is the most electronegative element. Learn how to find electronegativity values, how they relate to bond. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. electronegativity is not a uniquely defined property and may depend on the definition. electronegativity is the tendency of an atom to attract electrons in a. Element With Highest Electronegativity.
From www.youtube.com
The element with highest electronegativity is YouTube Element With Highest Electronegativity electronegativity is the tendency of an atom to attract electrons in a bond. 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. Find out which element has. Learn how to find electronegativity values, how they relate to bond. 119 rows learn about electronegativity, its measurement, importance and trends. Element With Highest Electronegativity.
From hxeiqucfl.blob.core.windows.net
Electronegativity Chlorine Higher Than Sulfur at Ronald Bartlett blog Element With Highest Electronegativity electronegativity is the tendency of an atom to attract electrons in a bond. Find out which element has. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. electronegativity is not a uniquely defined property and may depend on the definition. 119 rows find out the electronegativity values of all the. Element With Highest Electronegativity.
From trendimage.netlify.app
Which Element Has The Highest Electronegativity Element With Highest Electronegativity of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. fluorine is the most electronegative element. The suggested values are all taken from. electronegativity is the tendency of an atom to attract electrons in a bond. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and. Element With Highest Electronegativity.
From www.sliderbase.com
Periodic Table of Electronegativities Element With Highest Electronegativity Learn how to find electronegativity values, how they relate to bond. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. fluorine is the most electronegative element. of the main group elements,. Element With Highest Electronegativity.
From quickanswer.org
Which element has the highest electronegativity? Element With Highest Electronegativity learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. The suggested values are all taken from. electronegativity is not a uniquely defined property and may depend on the definition. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en. Element With Highest Electronegativity.
From utedzz.blogspot.com
Printable Periodic Table With Electronegativity Values Periodic Table Element With Highest Electronegativity learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Learn how to find electronegativity values, how they relate to bond. 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. of the main group elements, fluorine has the highest. Element With Highest Electronegativity.
From wisc.pb.unizin.org
Bonding and Electronegativity (M8Q1) UWMadison Chemistry 103/104 Element With Highest Electronegativity 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. electronegativity is not a uniquely defined property and may depend on the definition. fluorine is the most electronegative element. Fluorine has an electronegativity. Element With Highest Electronegativity.
From utedzz.blogspot.com
Periodic Table With Electronegativity Periodic Table Timeline Element With Highest Electronegativity electronegativity is the tendency of an atom to attract electrons in a bond. electronegativity is not a uniquely defined property and may depend on the definition. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. 119 rows find out the electronegativity values of all the elements. Element With Highest Electronegativity.
From wisc.pb.unizin.org
M8Q1 Bonding and Electronegativity Chem 103/104 Resource Book Element With Highest Electronegativity 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. The suggested values are all taken from. Find out which element has. electronegativity is the tendency of an atom to attract electrons in a bond. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. fluorine is. Element With Highest Electronegativity.
From mavink.com
Periodic Table With Electronegativity Values Element With Highest Electronegativity The suggested values are all taken from. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a. Element With Highest Electronegativity.
From sciencenotes.org
Electronegativity Chart PDF Element With Highest Electronegativity Find out which element has. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. electronegativity is not a uniquely defined property and may depend on the definition. Learn how to find electronegativity values, how they relate to bond. fluorine is the most electronegative element. learn how to define and measure. Element With Highest Electronegativity.
From socratic.org
Which group of elements is listed in order of increasing Element With Highest Electronegativity of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. Find out which element has. The suggested values are. Element With Highest Electronegativity.
From www.youtube.com
Which element has highest electronegativity? YouTube Element With Highest Electronegativity Find out which element has. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Learn how to find electronegativity values, how they relate to bond. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. 119 rows learn about electronegativity, its measurement,. Element With Highest Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Element With Highest Electronegativity 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. Learn how to find electronegativity. Element With Highest Electronegativity.
From socratic.org
Periodic Trends in Electronegativity Chemistry Socratic Element With Highest Electronegativity 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. The suggested values are all taken from. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. fluorine is the most electronegative element. of the main group elements, fluorine has the highest electronegativity. Element With Highest Electronegativity.
From utedzz.blogspot.com
Periodic Table With Electronegativity Printable Periodic Table Timeline Element With Highest Electronegativity 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. fluorine is the most electronegative element. Find out which element has. Learn how to find electronegativity values, how they relate to bond. electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all taken from. . Element With Highest Electronegativity.
From fluxdesignhouse.blogspot.com
How To Find Electronegativity Of An Element fluxdesignhouse Element With Highest Electronegativity of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. The suggested values are all taken from. 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. electronegativity is not a uniquely defined property and may depend on the definition.. Element With Highest Electronegativity.
From sciencetrends.com
Electronegativity Chart Science Trends Element With Highest Electronegativity electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all taken from. electronegativity is the tendency of an atom to attract electrons in a bond. fluorine is the most electronegative element. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. Find out. Element With Highest Electronegativity.
From www.youtube.com
Element with highest electronegativity electronegativity SR chemistry Element With Highest Electronegativity learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Learn how to find electronegativity values, how they relate to bond. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. electronegativity is not a uniquely defined property and may depend on the definition.. Element With Highest Electronegativity.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps Element With Highest Electronegativity Find out which element has. 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. electronegativity is the tendency of an atom to attract electrons in a bond. Learn how. Element With Highest Electronegativity.
From dona.tompkinscountystructuralracism.org
Electronegativity Values Chart Understanding The Chemistry Of Elements Element With Highest Electronegativity Learn how to find electronegativity values, how they relate to bond. electronegativity is the tendency of an atom to attract electrons in a bond. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. 119 rows find out the electronegativity values of all the elements in the periodic. Element With Highest Electronegativity.
From knordslearning.com
Electronegativity Chart of all Elements (With Periodic table) Element With Highest Electronegativity fluorine is the most electronegative element. Learn how to find electronegativity values, how they relate to bond. of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. 119. Element With Highest Electronegativity.
From mavink.com
Periodic Table With En Values Element With Highest Electronegativity fluorine is the most electronegative element. electronegativity is not a uniquely defined property and may depend on the definition. 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound.. Element With Highest Electronegativity.
From www.britannica.com
Chemical compound Trends, Elements, Properties Britannica Element With Highest Electronegativity The suggested values are all taken from. fluorine is the most electronegative element. electronegativity is the tendency of an atom to attract electrons in a bond. 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. electronegativity is not a uniquely defined property and may depend on the. Element With Highest Electronegativity.
From www.sliderbase.com
Periodic Table of Electronegativities Element With Highest Electronegativity of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. Find out which element has. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. Learn how to find electronegativity values, how they relate to bond. The suggested values are all taken from. fluorine. Element With Highest Electronegativity.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Element With Highest Electronegativity 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. Find out which element has. Learn how to find electronegativity values, how they relate to bond. 119 rows learn about electronegativity, its measurement, importance. Element With Highest Electronegativity.
From anthonystrendsassignment.weebly.com
Electronegativity Periodic Trends Element With Highest Electronegativity of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. The suggested values are all taken from. Find out which element has. electronegativity is not a uniquely defined property and may depend on the definition. learn how to define and measure electronegativity, the ability of an atom to. Element With Highest Electronegativity.
From www.angelo.edu
The Parts of the Periodic Table Element With Highest Electronegativity electronegativity is the tendency of an atom to attract electrons in a bond. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale. Element With Highest Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Element With Highest Electronegativity of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. Learn how to find electronegativity values, how they relate to bond. Find out which element has. fluorine is the most electronegative element. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. . Element With Highest Electronegativity.
From www.slideserve.com
PPT Electronegativity? PowerPoint Presentation, free download ID Element With Highest Electronegativity of the main group elements, fluorine has the highest electronegativity (en \(= 4.0\)) and cesium the lowest (en \(=. Find out which element has. 119 rows find out the electronegativity values of all the elements in the periodic table, from hydrogen to. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. . Element With Highest Electronegativity.
From utedzz.blogspot.com
Periodic Table Electronegativity Values Periodic Table Timeline Element With Highest Electronegativity Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. Learn how to find electronegativity values, how they relate to bond. fluorine is the most electronegative element. 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. of the main group elements, fluorine has the highest electronegativity. Element With Highest Electronegativity.
From www.pinterest.com
Periodic Table Electronegativity Trend Ionization energy Element With Highest Electronegativity 119 rows learn about electronegativity, its measurement, importance and trends in the periodic table. electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all taken from. Fluorine has an electronegativity of 3.98 on the pauling electronegativity scale and a valence of 1. Learn how to find electronegativity values, how they. Element With Highest Electronegativity.