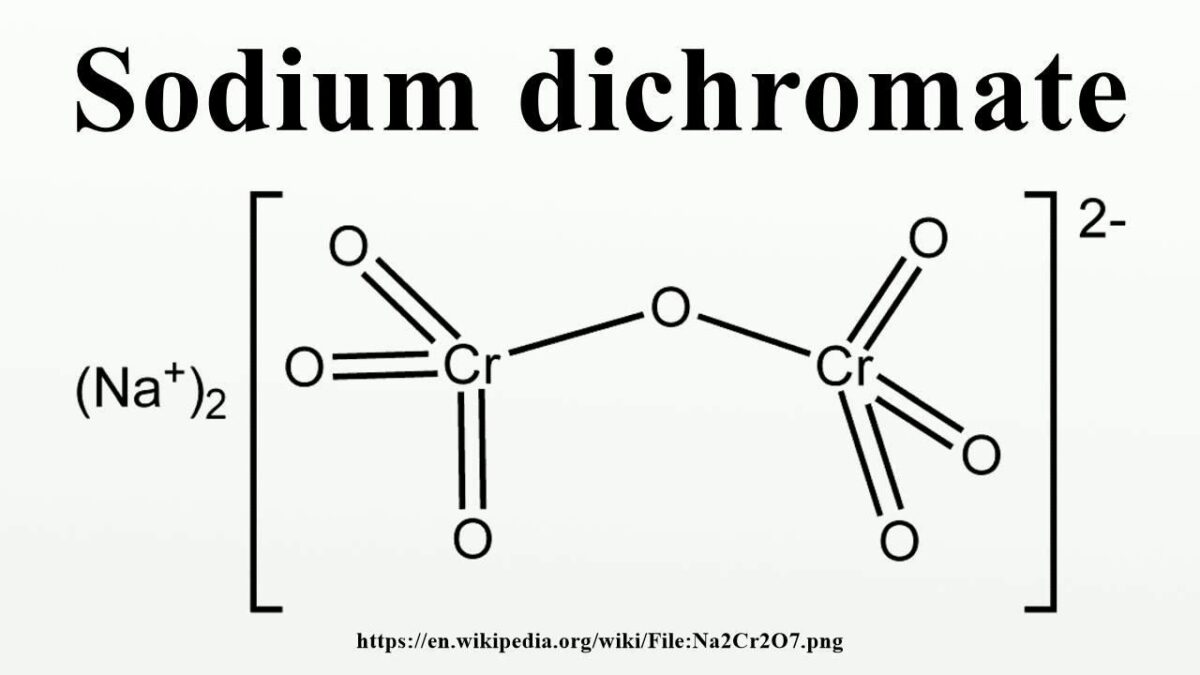
Zinc Dichromate Ion Charge . zinc (2+) ion chromate. We need three f − ions to balance the. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. For example, iron( ii ) has a 2+ charge;. start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. Using equations to represent chemical reactions working out the charges of ions. Because the overall compound must be. All substances are described by their formulae, which are. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. roman numeral notation indicates charge of ion when element commonly forms more than one ion.
from h-o-m-e.org
start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. roman numeral notation indicates charge of ion when element commonly forms more than one ion. zinc (2+) ion chromate. We need three f − ions to balance the. dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. All substances are described by their formulae, which are. Using equations to represent chemical reactions working out the charges of ions. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). Because the overall compound must be.
Dichromate Formula, Facts And FAQ
Zinc Dichromate Ion Charge zinc (2+) ion chromate. For example, iron( ii ) has a 2+ charge;. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. roman numeral notation indicates charge of ion when element commonly forms more than one ion. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). Because the overall compound must be. We need three f − ions to balance the. All substances are described by their formulae, which are. start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. Using equations to represent chemical reactions working out the charges of ions. dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. zinc (2+) ion chromate.
From www.sarthaks.com
Draw the structures of chromate and dichromate ions. Sarthaks Zinc Dichromate Ion Charge dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. start by writing the metal ion with its charge, followed by the. Zinc Dichromate Ion Charge.
From www.youtube.com
Ionic Charge for Zinc (Zn) YouTube Zinc Dichromate Ion Charge We need three f − ions to balance the. dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. roman numeral notation indicates. Zinc Dichromate Ion Charge.
From www.youtube.com
How to find the Oxidation Number for Cr in the Cr2O7 2 ion Zinc Dichromate Ion Charge We need three f − ions to balance the. For example, iron( ii ) has a 2+ charge;. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. roman numeral notation indicates charge of ion when element commonly forms more than one ion. Using. Zinc Dichromate Ion Charge.
From slideplayer.com
Ionic Compounds Naming and Writing Formulas ppt download Zinc Dichromate Ion Charge zinc (2+) ion chromate. roman numeral notation indicates charge of ion when element commonly forms more than one ion. All substances are described by their formulae, which are. start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. We need three f − ions to balance the. For example, iron(. Zinc Dichromate Ion Charge.
From www.youtube.com
How to find the Oxidation Number for Cr in the CrO4 2 ion. (Chromate Zinc Dichromate Ion Charge For example, iron( ii ) has a 2+ charge;. All substances are described by their formulae, which are. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then. Zinc Dichromate Ion Charge.
From www.nanochemazone.com
Zinc Dichromate Trihydrate Powder Low Price 1 Nanochemazone Zinc Dichromate Ion Charge we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. We need three f − ions to balance the. For example, iron( ii ) has a 2+. Zinc Dichromate Ion Charge.
From www.slideshare.net
Writing More Complex Redox Equations Zinc Dichromate Ion Charge For example, iron( ii ) has a 2+ charge;. dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. zinc (2+) ion chromate. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to. Zinc Dichromate Ion Charge.
From www.toppr.com
Write the net ionic equation for the reaction of potassium dichromate Zinc Dichromate Ion Charge dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. Using equations to represent chemical reactions working out the charges of ions. For example, iron( ii. Zinc Dichromate Ion Charge.
From www.pdfprof.com
ion cu+ Zinc Dichromate Ion Charge zinc (2+) ion chromate. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). We need three f − ions to balance the. start. Zinc Dichromate Ion Charge.
From askfilo.com
Identify the correct structure of dichromate ion. Filo Zinc Dichromate Ion Charge Using equations to represent chemical reactions working out the charges of ions. All substances are described by their formulae, which are. For example, iron( ii ) has a 2+ charge;. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. dichromate(vi) ions (for example,. Zinc Dichromate Ion Charge.
From askfilo.com
(ii) I he chromate ion is tetrahedral whereas the dichromate ion consists.. Zinc Dichromate Ion Charge start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. zinc (2+) ion chromate. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). Using equations to represent chemical reactions working out the charges of ions. Because the overall compound must be.. Zinc Dichromate Ion Charge.
From utedzz.blogspot.com
Periodic Table Zinc Charge Periodic Table Timeline Zinc Dichromate Ion Charge dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). zinc (2+) ion chromate. we need two cl − ions to. Zinc Dichromate Ion Charge.
From h-o-m-e.org
Dichromate Formula, Facts And FAQ Zinc Dichromate Ion Charge dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. Using equations to represent chemical reactions working out the charges of ions. All substances are described by their formulae, which are. zinc (2+) ion chromate. we need two cl −. Zinc Dichromate Ion Charge.
From www.youtube.com
Structure Of Chromate Ion And Dichromate Ion D and F Block Elements Zinc Dichromate Ion Charge start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. Because the overall compound must be. dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. we need two cl − ions to. Zinc Dichromate Ion Charge.
From www.youtube.com
STRUCTURE OF CHROMATE AND DICHROMATE ION, DRUNK TEST YouTube Zinc Dichromate Ion Charge All substances are described by their formulae, which are. zinc (2+) ion chromate. We need three f − ions to balance the. roman numeral notation indicates charge of ion when element commonly forms more than one ion. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic. Zinc Dichromate Ion Charge.
From sansona.github.io
Polyatomic Ions Zinc Dichromate Ion Charge All substances are described by their formulae, which are. Because the overall compound must be. For example, iron( ii ) has a 2+ charge;. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. Using equations to represent chemical reactions working out the charges of. Zinc Dichromate Ion Charge.
From www.numerade.com
SOLVEDThe dichromate ion, Cr2 O7^2, has neither CrCr nor OO bonds Zinc Dichromate Ion Charge Because the overall compound must be. zinc (2+) ion chromate. All substances are described by their formulae, which are. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to. Zinc Dichromate Ion Charge.
From quizlet.com
The dichromate ion, \mathrm{Cr}_2 \mathrm{O}_7^{2}, has n Quizlet Zinc Dichromate Ion Charge dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). Using equations to represent chemical reactions working out the charges of ions. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. For example, iron( ii. Zinc Dichromate Ion Charge.
From www.numerade.com
SOLVED When dichromate ion is reacted with zinc metal under acidic Zinc Dichromate Ion Charge Using equations to represent chemical reactions working out the charges of ions. zinc (2+) ion chromate. All substances are described by their formulae, which are. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. We need three f − ions to balance the.. Zinc Dichromate Ion Charge.
From www.gauthmath.com
Solved What product forms when zinc reacts with sulfur? Hint Remember Zinc Dichromate Ion Charge start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. For example, iron( ii ) has a 2+ charge;. dichromate (vi) ions (for example, in potassium. Zinc Dichromate Ion Charge.
From www.pinterest.com
Ion Names, Formulas and Charges Chart Flinn Scientific Chemistry Zinc Dichromate Ion Charge Because the overall compound must be. dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. For example, iron( ii ) has a 2+ charge;. Using equations to represent chemical reactions working out the charges of ions. zinc (2+) ion chromate.. Zinc Dichromate Ion Charge.
From www.slideserve.com
PPT Lecture 19. The d Block Elements. IVVI B groups PowerPoint Zinc Dichromate Ion Charge For example, iron( ii ) has a 2+ charge;. Because the overall compound must be. roman numeral notation indicates charge of ion when element commonly forms more than one ion. We need three f − ions to balance the. zinc (2+) ion chromate. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions. Zinc Dichromate Ion Charge.
From valenceelectrons.com
Complete Electron Configuration for Zinc (Zn, Zn2+ ion) Zinc Dichromate Ion Charge dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. zinc (2+) ion chromate. start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. Using equations to represent chemical reactions working out the. Zinc Dichromate Ion Charge.
From utedzz.blogspot.com
Periodic Table Zinc Charge Periodic Table Timeline Zinc Dichromate Ion Charge start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. For example, iron( ii ) has a 2+ charge;. roman numeral notation indicates charge of ion when element commonly forms more than one ion. Because the overall compound must be. All substances are described by their formulae, which are. dichromate. Zinc Dichromate Ion Charge.
From www.sliderbase.com
Naming compounds and ions Presentation Chemistry Zinc Dichromate Ion Charge We need three f − ions to balance the. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. Using equations to represent chemical reactions working. Zinc Dichromate Ion Charge.
From www.chegg.com
Solved Dichromate Ion Is An Oxidizing Agent In Acidic Sol... Zinc Dichromate Ion Charge start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. For example, iron( ii ) has a 2+ charge;. dichromate(vi) ions (for example, in potassium dichromate(vi). Zinc Dichromate Ion Charge.
From www.alamy.com
benzene Stock Vector Images Alamy Zinc Dichromate Ion Charge Because the overall compound must be. start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. All substances are described by their formulae, which are. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). dichromate (vi) ions (for example, in potassium. Zinc Dichromate Ion Charge.
From www.science-revision.co.uk
Further redox reactions Zinc Dichromate Ion Charge For example, iron( ii ) has a 2+ charge;. zinc (2+) ion chromate. roman numeral notation indicates charge of ion when element commonly forms more than one ion. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). Using equations to represent chemical reactions working out the charges of. Zinc Dichromate Ion Charge.
From chemistryfromscratch.org
V3.9 Zinc Dichromate Ion Charge we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). Because the overall compound must be. For example, iron( ii ) has a 2+ charge;. Using. Zinc Dichromate Ion Charge.
From www.youtube.com
Finding the charge of a Transition Metal ion in a compound National 5 Zinc Dichromate Ion Charge We need three f − ions to balance the. All substances are described by their formulae, which are. For example, iron( ii ) has a 2+ charge;. dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. Using equations to represent chemical. Zinc Dichromate Ion Charge.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Zinc Dichromate Ion Charge Using equations to represent chemical reactions working out the charges of ions. Because the overall compound must be. roman numeral notation indicates charge of ion when element commonly forms more than one ion. We need three f − ions to balance the. For example, iron( ii ) has a 2+ charge;. start by writing the metal ion with. Zinc Dichromate Ion Charge.
From www.youtube.com
How to Write the Formula for Zinc dichromate YouTube Zinc Dichromate Ion Charge roman numeral notation indicates charge of ion when element commonly forms more than one ion. We need three f − ions to balance the. we need two cl − ions to balance the charge on one ca 2 + ion, so the proper ionic formula is cacl 2. Because the overall compound must be. For example, iron( ii. Zinc Dichromate Ion Charge.
From www.researchgate.net
Scheme of the simulated zincion battery. Download Scientific Diagram Zinc Dichromate Ion Charge dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). All substances are described by their formulae, which are. We need three f − ions to balance the. zinc (2+) ion chromate. Using equations to represent chemical reactions working out the charges of ions. Because the overall compound must be.. Zinc Dichromate Ion Charge.
From www.sarthaks.com
Identify the correct structure of dichromate ion. Sarthaks eConnect Zinc Dichromate Ion Charge We need three f − ions to balance the. Because the overall compound must be. dichromate (vi) ions (for example, in potassium dichromate (vi) solution) can be reduced to chromium (iii) ions and then to chromium (ii) ions using zinc and either. we need two cl − ions to balance the charge on one ca 2 + ion,. Zinc Dichromate Ion Charge.
From www.youtube.com
Redox Reaction dichromate ion with sulphite ion in acidic medium Zinc Dichromate Ion Charge start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. We need three f − ions to balance the. zinc (2+) ion chromate. dichromate(vi) ions (for example, in potassium dichromate(vi) solution) can be reduced to chromium(iii) ions and then to chromium(ii). Using equations to represent chemical reactions working out the. Zinc Dichromate Ion Charge.