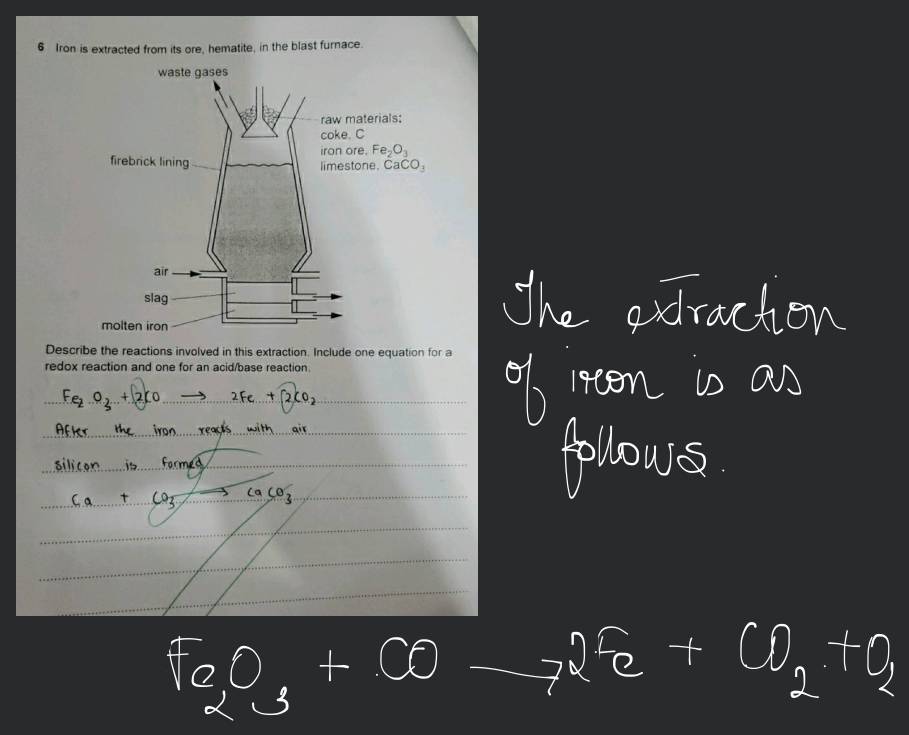
Iron Is Extracted From Its Ore Hematite In A Blast Furnace . 5 iron is extracted from its ore, hematite, in a blast furnace. The furnace is filled at the. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. the production of iron from its ore involves a redox reaction carried out in a blast furnace. Air firebrick lining raw materials:: 5 iron is extracted from its ore, hematite, in the blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Coke, c iron ore, fe 2o 3. We’ll look at the chemicals used in the blast furnace, and the reactions. in this video, we will learn about the extraction of iron from its ore in the blast furnace. Substances added to the furnace are: Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the.
from askfilo.com
the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. Air firebrick lining raw materials:: 5 iron is extracted from its ore, hematite, in a blast furnace. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. The furnace is filled at the. in this video, we will learn about the extraction of iron from its ore in the blast furnace. the production of iron from its ore involves a redox reaction carried out in a blast furnace. Coke, c iron ore, fe 2o 3. Substances added to the furnace are: 5 iron is extracted from its ore, hematite, in the blast furnace.
6 Iron is extracted from its ore, hematite, in the blast furnace.Describ..
Iron Is Extracted From Its Ore Hematite In A Blast Furnace Substances added to the furnace are: The furnace is filled at the. in this video, we will learn about the extraction of iron from its ore in the blast furnace. 5 iron is extracted from its ore, hematite, in a blast furnace. Coke, c iron ore, fe 2o 3. the production of iron from its ore involves a redox reaction carried out in a blast furnace. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. Substances added to the furnace are: We’ll look at the chemicals used in the blast furnace, and the reactions. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Air firebrick lining raw materials:: Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. 5 iron is extracted from its ore, hematite, in the blast furnace.
From askfilo.com
6 Iron is extracted from its ore, hematite, in the blast furnace.Describ.. Iron Is Extracted From Its Ore Hematite In A Blast Furnace The furnace is filled at the. the production of iron from its ore involves a redox reaction carried out in a blast furnace. Substances added to the furnace are: the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. 5 iron is extracted from its. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.blendspace.com
Extraction Of Iron Blast Furnace Lessons Blendspace Iron Is Extracted From Its Ore Hematite In A Blast Furnace Substances added to the furnace are: in this video, we will learn about the extraction of iron from its ore in the blast furnace. 5 iron is extracted from its ore, hematite, in a blast furnace. We’ll look at the chemicals used in the blast furnace, and the reactions. Redox reactions are involved in the extraction of metals. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.tuttee.co
CHEM Extraction of Metal chemistry metal extraction blast furnace electrolysis Iron Is Extracted From Its Ore Hematite In A Blast Furnace in this video, we will learn about the extraction of iron from its ore in the blast furnace. 5 iron is extracted from its ore, hematite, in the blast furnace. the production of iron from its ore involves a redox reaction carried out in a blast furnace. the extraction of iron from its ore is a. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.23C Explain How the Method of Extraction of a Metal is Related to its Iron Is Extracted From Its Ore Hematite In A Blast Furnace We’ll look at the chemicals used in the blast furnace, and the reactions. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. the production of iron from its ore involves a redox reaction carried out in a blast furnace. revision notes on extraction of iron from hematite for. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.pinterest.com
THE BLAST FURNACE IRON PRODUCTION Refractory Brick, Iron Ore, Information Processing Iron Is Extracted From Its Ore Hematite In A Blast Furnace Coke, c iron ore, fe 2o 3. Air firebrick lining raw materials:: Substances added to the furnace are: the production of iron from its ore involves a redox reaction carried out in a blast furnace. The furnace is filled at the. 5 iron is extracted from its ore, hematite, in a blast furnace. We’ll look at the chemicals. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From app.pandai.org
Extraction of Metals from its Ore Iron Is Extracted From Its Ore Hematite In A Blast Furnace 5 iron is extracted from its ore, hematite, in a blast furnace. Substances added to the furnace are: the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.engineeringclicks.com
A Quick Guide to Ferrous Metals EngineeringClicks Iron Is Extracted From Its Ore Hematite In A Blast Furnace Air firebrick lining raw materials:: Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. in this video, we will learn about the extraction of iron from its ore in the blast furnace. the extraction of iron from its ore is a long and subdued process, that helps in. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.vedantu.com
The flux used in the extraction of iron from haematite ore in the blast furnace isA.LimestoneB Iron Is Extracted From Its Ore Hematite In A Blast Furnace Substances added to the furnace are: Coke, c iron ore, fe 2o 3. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. Air firebrick lining raw materials:: the production of iron from its ore involves a redox reaction carried out in a blast furnace. the extraction of iron. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From askfilo.com
6 Iron is extracted from its ore, hematite, in the blast furnace.Describ.. Iron Is Extracted From Its Ore Hematite In A Blast Furnace 5 iron is extracted from its ore, hematite, in the blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. Coke, c iron ore,. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From chem.libretexts.org
23.3 Metallurgy of Iron and Steel Chemistry LibreTexts Iron Is Extracted From Its Ore Hematite In A Blast Furnace the production of iron from its ore involves a redox reaction carried out in a blast furnace. in this video, we will learn about the extraction of iron from its ore in the blast furnace. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. 5 iron is. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From dxodcnsin.blob.core.windows.net
Iron Is Extracted From Haematite Ore By at June McDonough blog Iron Is Extracted From Its Ore Hematite In A Blast Furnace Air firebrick lining raw materials:: Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. The furnace is filled at the. 5 iron is extracted from its ore, hematite, in the blast furnace. Coke, c iron ore, fe 2o 3. Substances added to the furnace are: We’ll look at the. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.youtube.com
Iron extraction YouTube Iron Is Extracted From Its Ore Hematite In A Blast Furnace revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Coke, c iron ore, fe 2o 3. 5 iron is extracted from its ore, hematite, in a blast furnace. 5 iron is extracted from its ore, hematite, in the blast furnace. Redox reactions are. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.greatmining.com
Iron Ore Mining Techniques Metal Extraction Iron Is Extracted From Its Ore Hematite In A Blast Furnace Air firebrick lining raw materials:: Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. 5 iron is extracted from its ore, hematite, in the blast furnace. The furnace is filled at the. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From saylordotorg.github.io
Metallurgy Iron Is Extracted From Its Ore Hematite In A Blast Furnace We’ll look at the chemicals used in the blast furnace, and the reactions. Coke, c iron ore, fe 2o 3. Air firebrick lining raw materials:: revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. The furnace is filled at the. Substances added to the furnace. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From y9mschemistry.blogspot.com
Year 9 Chemistry 2012 Extraction of iron from its ores Iron Is Extracted From Its Ore Hematite In A Blast Furnace 5 iron is extracted from its ore, hematite, in the blast furnace. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. Substances added to the furnace. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.vedantu.com
Draw a neat diagram of the blast furnace used in the extraction of iron and label the parts. Iron Is Extracted From Its Ore Hematite In A Blast Furnace The furnace is filled at the. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. Coke, c iron ore, fe 2o 3. the production of iron from its ore involves a redox reaction carried out in a blast furnace. revision notes on extraction of. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From ukcsma.co.uk
csmaBlastFurnaceDiagram CSMA The Cementitious Slag Makers Association Iron Is Extracted From Its Ore Hematite In A Blast Furnace 5 iron is extracted from its ore, hematite, in the blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. 5 iron is extracted from its ore, hematite, in a blast furnace. Redox reactions are involved in the extraction of metals from. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.w3schools.blog
Iron Extraction W3schools Iron Is Extracted From Its Ore Hematite In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. The furnace is filled at the. the production of iron from its ore involves a redox reaction carried out in a blast furnace. 5 iron is extracted from its ore, hematite, in the blast furnace.. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.sliderbase.com
Blast Furnace Presentation Chemistry Iron Is Extracted From Its Ore Hematite In A Blast Furnace Coke, c iron ore, fe 2o 3. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. We’ll look at the chemicals used in the blast furnace, and. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.savemyexams.co.uk
Extraction of Iron from Hematite (9.3.2) CIE IGCSE Chemistry Revision Notes 2023 Save My Exams Iron Is Extracted From Its Ore Hematite In A Blast Furnace The furnace is filled at the. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. We’ll look at the chemicals used in the blast furnace, and the reactions. Coke, c iron ore, fe 2o 3. 5 iron is extracted from its ore, hematite, in a blast furnace. Air firebrick. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.w3schools.blog
Iron Extraction W3schools Iron Is Extracted From Its Ore Hematite In A Blast Furnace Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. the production of iron from its ore involves a redox reaction carried out in a blast furnace. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the.. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.slideshare.net
Extraction Of Iron Iron Is Extracted From Its Ore Hematite In A Blast Furnace 5 iron is extracted from its ore, hematite, in the blast furnace. the production of iron from its ore involves a redox reaction carried out in a blast furnace. We’ll look at the chemicals used in the blast furnace, and the reactions. Substances added to the furnace are: Air firebrick lining raw materials:: Redox reactions are involved in. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.numerade.com
SOLVED The reduction of iron ore, hematite, takes place in the blast furnace for the extraction Iron Is Extracted From Its Ore Hematite In A Blast Furnace Substances added to the furnace are: Air firebrick lining raw materials:: Coke, c iron ore, fe 2o 3. 5 iron is extracted from its ore, hematite, in a blast furnace. The furnace is filled at the. the production of iron from its ore involves a redox reaction carried out in a blast furnace. in this video, we. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.youtube.com
Extraction of Metals from Ores with basic concepts and Explanation with examples YouTube Iron Is Extracted From Its Ore Hematite In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. The furnace is filled at the. We’ll look at the chemicals used in the blast furnace, and the reactions. 5 iron is extracted from its ore, hematite, in the blast furnace. 5 iron is extracted. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.youtube.com
Extraction of Iron Making Iron in the Blast Furnace teachyourselfchemistry studyfromhome Iron Is Extracted From Its Ore Hematite In A Blast Furnace in this video, we will learn about the extraction of iron from its ore in the blast furnace. Air firebrick lining raw materials:: the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. Coke, c iron ore, fe 2o 3. Substances added to the furnace are:. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.vecteezy.com
Blast Furnace for the smelting of iron ore, Metallurgy of iron and steel 18891989 Vector Art at Iron Is Extracted From Its Ore Hematite In A Blast Furnace Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. in this video, we will learn about the extraction of iron from its ore in the blast furnace. the production of iron from its ore involves a redox reaction carried out in a blast furnace. the extraction of. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.chegg.com
Solved Q.2) Iron is extracted from its ore, hematite, in the Iron Is Extracted From Its Ore Hematite In A Blast Furnace in this video, we will learn about the extraction of iron from its ore in the blast furnace. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. 5 iron is extracted from its ore, hematite, in a blast furnace. Coke, c iron ore, fe. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From byjus.com
How is iron extracted from hematite? Iron Is Extracted From Its Ore Hematite In A Blast Furnace We’ll look at the chemicals used in the blast furnace, and the reactions. Air firebrick lining raw materials:: 5 iron is extracted from its ore, hematite, in a blast furnace. in this video, we will learn about the extraction of iron from its ore in the blast furnace. the production of iron from its ore involves a. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From studyinggcsechem.blogspot.com
IGCSE Chemistry 5.4 Describe and explain the main reactions involved in the extraction of iron Iron Is Extracted From Its Ore Hematite In A Blast Furnace Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. in this video, we will learn about the extraction of iron from its ore in the blast furnace. 5 iron is extracted from its ore, hematite, in the blast furnace. The furnace is filled at the. We’ll look at. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From studyinggcsechem.blogspot.com
IGCSE Chemistry 5.4 Describe and explain the main reactions involved in the extraction of iron Iron Is Extracted From Its Ore Hematite In A Blast Furnace Substances added to the furnace are: the production of iron from its ore involves a redox reaction carried out in a blast furnace. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From classnotes.org.in
Extraction of Iron Class 12, General Principles and Processes of Isolation of Elements Iron Is Extracted From Its Ore Hematite In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. Substances added to the furnace are: Air firebrick lining raw materials:: the production of iron from its ore involves a redox reaction carried out in a blast furnace. 5 iron is extracted from its ore,. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.vedantu.com
Draw the diagram of the blast furnace used in the extraction of iron and label the molten iron. Iron Is Extracted From Its Ore Hematite In A Blast Furnace the production of iron from its ore involves a redox reaction carried out in a blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. We’ll look at the chemicals used in the blast furnace, and the reactions. The furnace is filled at. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.slideshare.net
Extraction Of Iron Iron Is Extracted From Its Ore Hematite In A Blast Furnace We’ll look at the chemicals used in the blast furnace, and the reactions. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the production of iron from its ore involves a redox reaction carried out in a blast furnace. Air firebrick lining raw materials::. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From dxodcnsin.blob.core.windows.net
Iron Is Extracted From Haematite Ore By at June McDonough blog Iron Is Extracted From Its Ore Hematite In A Blast Furnace Coke, c iron ore, fe 2o 3. in this video, we will learn about the extraction of iron from its ore in the blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Air firebrick lining raw materials:: Redox reactions are involved in. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.
From www.youtube.com
Iron is produced from its ore, hematite, Fe2O3, by heating with carbon monoxide in a blast Iron Is Extracted From Its Ore Hematite In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the. in this video, we will learn about the extraction of iron from its ore in the blast furnace. The furnace is filled at the. the production of iron from its ore involves a redox reaction. Iron Is Extracted From Its Ore Hematite In A Blast Furnace.