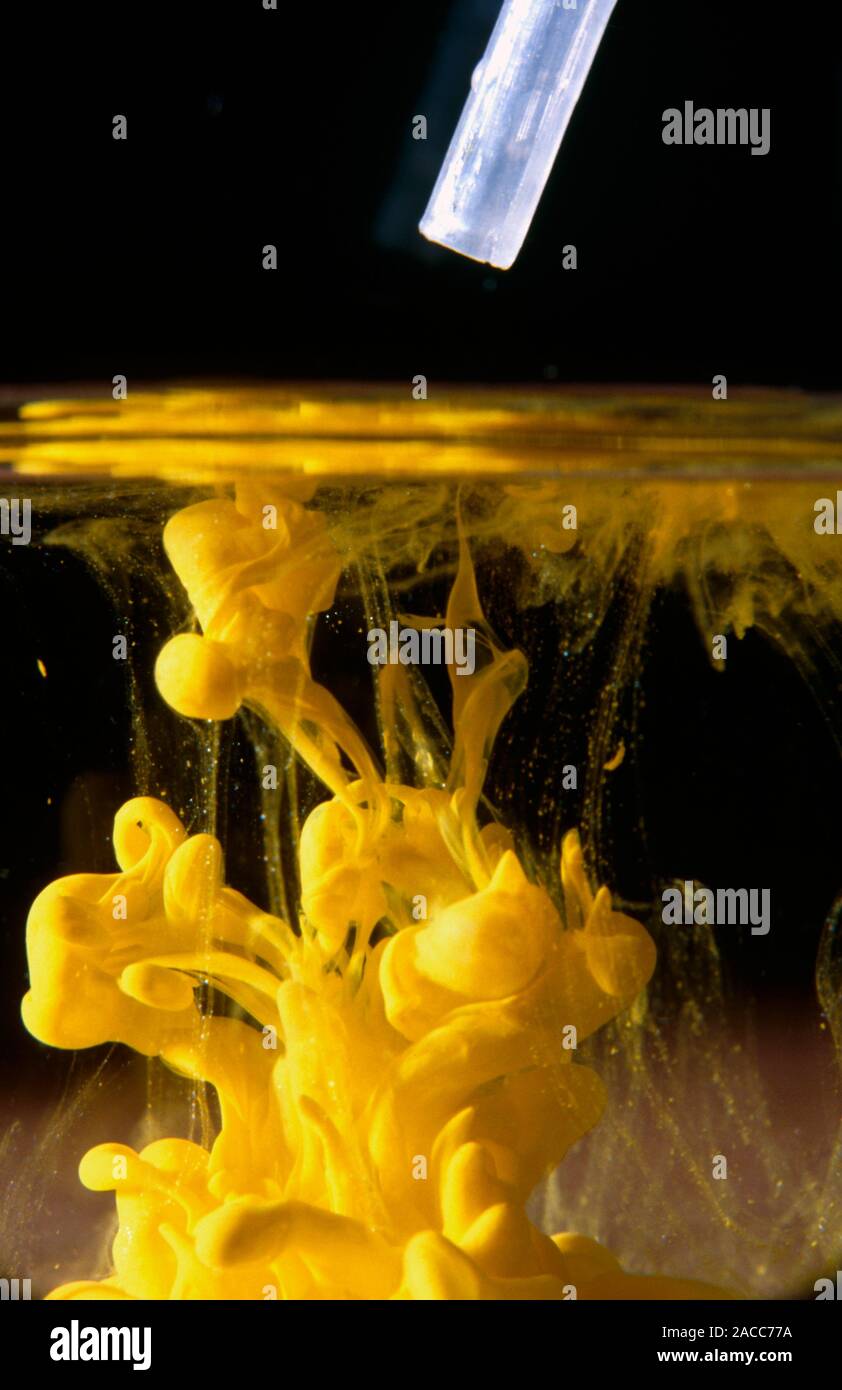
Lead Iodide Potassium Nitrate . use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules.
from
learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate.
Lead Iodide Potassium Nitrate lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead.
From
Lead Iodide Potassium Nitrate learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). use this demonstration with kit list and safety instructions to prove that two solids can react. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. Find out how to do the experiment, what happens when you heat or cool the solution, and. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. use this demonstration with kit list and safety instructions to prove. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. Find out how to do the experiment, what happens when you heat or. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. Find out how to do the experiment, what happens. Lead Iodide Potassium Nitrate.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Lead Iodide Potassium Nitrate lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. learn how to calculate the number of moles. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. learn how to predict. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. Find out how to do the experiment, what happens when you heat or cool the solution, and. Lead Iodide Potassium Nitrate.
From www.youtube.com
Lead Nitrate and Potassium Iodide Reaction Activity 1.2 CBSE 10 Lead Iodide Potassium Nitrate learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. learn how to calculate. Lead Iodide Potassium Nitrate.
From www.youtube.com
REACTION OF LEAD NITRATE WITH POTASSIUM IODIDE YouTube Lead Iodide Potassium Nitrate Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki). Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead. Lead Iodide Potassium Nitrate.
From www.numerade.com
SOLVED 'KI + Pb(NO3)2 → Pbl2 + KNO3NThis chemical reaction Lead Iodide Potassium Nitrate learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to calculate the number of moles. Lead Iodide Potassium Nitrate.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Lead Iodide Potassium Nitrate learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi. Lead Iodide Potassium Nitrate.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Potassium Iodide YouTube Lead Iodide Potassium Nitrate learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. Find out how to do the experiment, what happens when you heat or cool the solution,. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of. Lead Iodide Potassium Nitrate.
From www.youtube.com
Demonstration Precipitation Reaction of Potassium Iodide and Lead Lead Iodide Potassium Nitrate lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. lead(ii) nitrate. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow. Lead Iodide Potassium Nitrate.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Lead Iodide Potassium Nitrate learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. Find out how to do the experiment, what happens when you heat or cool the solution,. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. Find out how to do the experiment, what happens when you heat. Lead Iodide Potassium Nitrate.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation ID5319064 Lead Iodide Potassium Nitrate learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. . Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to calculate the number of moles and mass of products in double replacement. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules.. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react. use this demonstration with kit list and safety instructions to prove that two solids can. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Find out how to do the experiment, what happens when. Lead Iodide Potassium Nitrate.
From www.alamy.com
Precipitation of lead iodide. A yellow precipitate of lead iodide Lead Iodide Potassium Nitrate lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. Find out how to do the experiment, what happens when you heat or cool the solution, and why solids can react.. Lead Iodide Potassium Nitrate.
From www.studypool.com
SOLUTION Reaction of potassium iodide with lead nitrate Studypool Lead Iodide Potassium Nitrate learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Find. Lead Iodide Potassium Nitrate.
From
Lead Iodide Potassium Nitrate learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. learn how to calculate the number of moles and mass of products in double replacement reactions, such as lead. use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate. Lead Iodide Potassium Nitrate.
From www.alamy.com
solutions of lead nitrate and potassium iodide will react together to Lead Iodide Potassium Nitrate learn how to predict precipitation reactions and solubility of common inorganic compounds using solubility rules. learn how to mix lead nitrate and potassium iodide solutions or powders to create a yellow precipitate of lead iodide and a white precipitate of potassium nitrate. Find out how to do the experiment, what happens when you heat or cool the solution,. Lead Iodide Potassium Nitrate.