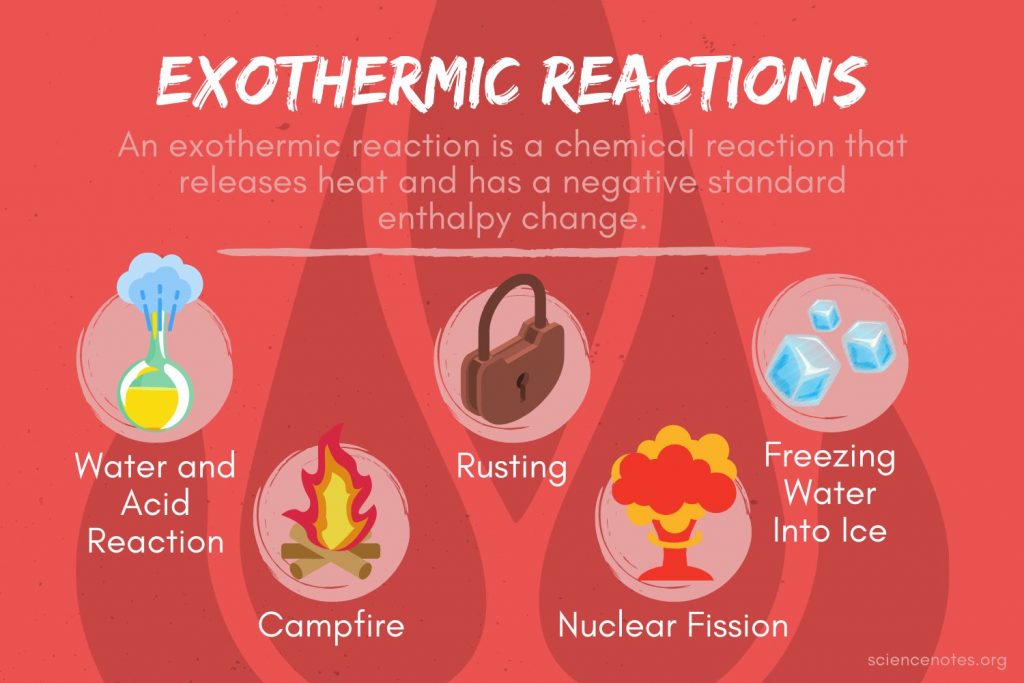
How To Tell If A Phase Change Is Endothermic Or Exothermic . There are two methods for distinguishing between exothermic and endothermic reactions. changes of state are examples of phase changes, or phase transitions. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Conversely, an exothermic reaction is. identifying exothermic & endothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. All phase changes are accompanied by changes in the energy of a system.
from classnotes123.com
changes of state are examples of phase changes, or phase transitions. There are two methods for distinguishing between exothermic and endothermic reactions. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. identifying exothermic & endothermic reactions. Conversely, an exothermic reaction is. All phase changes are accompanied by changes in the energy of a system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat.
What does one mean by exothermic and endothermic reactions? Give
How To Tell If A Phase Change Is Endothermic Or Exothermic the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. There are two methods for distinguishing between exothermic and endothermic reactions. changes of state are examples of phase changes, or phase transitions. identifying exothermic & endothermic reactions. Conversely, an exothermic reaction is. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). All phase changes are accompanied by changes in the energy of a system.
From www.researchgate.net
Endothermic or exothermic reaction of the VO 2 phase caused by phase How To Tell If A Phase Change Is Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Conversely, an exothermic reaction is. changes of state are examples of phase changes, or phase transitions. an endothermic reaction occurs when energy is absorbed from the surroundings. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From www.youtube.com
How to Determine if Phase Change is Endothermic or Exothermic Examples How To Tell If A Phase Change Is Endothermic Or Exothermic identifying exothermic & endothermic reactions. Conversely, an exothermic reaction is. There are two methods for distinguishing between exothermic and endothermic reactions. All phase changes are accompanied by changes in the energy of a system. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. endothermic reactions are. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! How To Tell If A Phase Change Is Endothermic Or Exothermic Conversely, an exothermic reaction is. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. There are two methods for distinguishing between exothermic and endothermic reactions. All phase changes are accompanied by changes in the. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From circuitengineragers88.z22.web.core.windows.net
Energy Diagram For An Endothermic Reaction How To Tell If A Phase Change Is Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. identifying exothermic & endothermic reactions. All phase changes are accompanied by changes in the energy of a system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). There are two methods for distinguishing between exothermic and endothermic reactions.. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE How To Tell If A Phase Change Is Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. Conversely, an exothermic reaction is. There are two methods for distinguishing between exothermic and endothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). changes of state are examples of phase changes, or phase transitions. . How To Tell If A Phase Change Is Endothermic Or Exothermic.
From lessonlibraryfettes.z22.web.core.windows.net
Endothermic Reactions And Exothermic Reaction How To Tell If A Phase Change Is Endothermic Or Exothermic endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). identifying exothermic & endothermic reactions. There are two methods for distinguishing between exothermic and endothermic reactions. Conversely, an exothermic reaction is. All phase changes are accompanied by changes in the energy of a system. an endothermic reaction occurs when energy. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From lessonlibraryfileting.z14.web.core.windows.net
Endothermic And Exothermic Explained How To Tell If A Phase Change Is Endothermic Or Exothermic Conversely, an exothermic reaction is. identifying exothermic & endothermic reactions. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. All phase changes are accompanied by changes in the energy of a system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From printableuzdisanje7n.z22.web.core.windows.net
Give 2 Examples Of Exothermic Reactions How To Tell If A Phase Change Is Endothermic Or Exothermic endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). There are two methods for distinguishing between exothermic and endothermic reactions. identifying exothermic & endothermic reactions. changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system.. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From eduinput.com
Difference Between Endothermic And Exothermic Reactions How To Tell If A Phase Change Is Endothermic Or Exothermic the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. changes of state are examples of phase changes, or phase transitions. identifying exothermic & endothermic reactions. All phase changes are accompanied by changes in the energy of a system. There are two methods for distinguishing between exothermic. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From circuitwiringpapes55.z22.web.core.windows.net
Energy Profile Of Endothermic Reaction How To Tell If A Phase Change Is Endothermic Or Exothermic the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Conversely, an exothermic reaction is. There are two methods for distinguishing between exothermic and endothermic reactions. identifying exothermic & endothermic reactions. All phase changes are accompanied by changes in the energy of a system. an endothermic reaction. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk How To Tell If A Phase Change Is Endothermic Or Exothermic endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). changes of state are examples of phase changes, or phase transitions. There are two methods for distinguishing between exothermic and endothermic reactions. the changes in energy that occur during a chemical reaction can be seen by examining the changes in. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From schematicdatavenin77.z5.web.core.windows.net
Endothermic And Exothermic Reaction Diagram How To Tell If A Phase Change Is Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. Conversely, an exothermic reaction is. All phase changes are accompanied by changes in the energy of a system. identifying exothermic & endothermic reactions. There are two methods for distinguishing between exothermic and endothermic reactions. an endothermic reaction occurs when energy is absorbed from the surroundings in. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From celaakhn.blob.core.windows.net
Endothermic Reaction Of Water at Griswold blog How To Tell If A Phase Change Is Endothermic Or Exothermic endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Conversely, an exothermic reaction is. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. All phase changes are accompanied by changes in the energy of a system. changes of state are examples of. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From schematicpaliza06.z22.web.core.windows.net
Exothermic And Endothermic Diagrams How To Tell If A Phase Change Is Endothermic Or Exothermic an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Conversely, an exothermic reaction is. identifying exothermic & endothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). There are two methods for distinguishing between exothermic and endothermic reactions. All phase changes. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From www.sexizpix.com
Ppt Endothermic Vs Exothermic Reaction Graphs Powerpoint Sexiz Pix How To Tell If A Phase Change Is Endothermic Or Exothermic Conversely, an exothermic reaction is. All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. There are two methods for distinguishing between exothermic and endothermic reactions. endothermic. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give How To Tell If A Phase Change Is Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. Conversely, an exothermic reaction is. changes of state are examples of phase changes, or phase transitions. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. the changes in energy that occur during a chemical reaction can be. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From circuitdiagramrefills.z21.web.core.windows.net
Chemistry Energy Diagrams How To Tell If A Phase Change Is Endothermic Or Exothermic There are two methods for distinguishing between exothermic and endothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). identifying exothermic & endothermic reactions. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. an endothermic reaction. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From educationdbaplikamz.z13.web.core.windows.net
The Change Of Water From One State To Another How To Tell If A Phase Change Is Endothermic Or Exothermic There are two methods for distinguishing between exothermic and endothermic reactions. identifying exothermic & endothermic reactions. changes of state are examples of phase changes, or phase transitions. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. All phase changes are accompanied by changes in the energy. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From printablemangers.z21.web.core.windows.net
Phase Changes And Phase Diagrams How To Tell If A Phase Change Is Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. Conversely, an exothermic reaction is. changes of state are examples of phase changes, or phase transitions. identifying exothermic & endothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the changes in energy that. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From quizizz.com
Printable endothermic and exothermic processes Worksheets for Year 10 How To Tell If A Phase Change Is Endothermic Or Exothermic an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. changes of state are examples of phase changes, or phase transitions. identifying exothermic & endothermic reactions. All phase changes are. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? How To Tell If A Phase Change Is Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. changes of state are examples of phase changes, or phase transitions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh).. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From learningbranjenjaq4.z13.web.core.windows.net
Heat And Phase Changes Worksheets How To Tell If A Phase Change Is Endothermic Or Exothermic identifying exothermic & endothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Conversely, an exothermic reaction is. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. changes of state are examples of phase changes, or phase transitions. the. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From cexdycgq.blob.core.windows.net
Why Does Temperature Affect The Kc at Lucille McDougall blog How To Tell If A Phase Change Is Endothermic Or Exothermic identifying exothermic & endothermic reactions. There are two methods for distinguishing between exothermic and endothermic reactions. changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. . How To Tell If A Phase Change Is Endothermic Or Exothermic.
From quizizz.com
50+ endothermic and exothermic processes worksheets for 11th Year on How To Tell If A Phase Change Is Endothermic Or Exothermic endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. All phase changes are accompanied by changes in the energy of a system. Conversely, an exothermic reaction is. identifying exothermic. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From printableabsolutefreakmn.z14.web.core.windows.net
Endothermic And Exothermic Explained How To Tell If A Phase Change Is Endothermic Or Exothermic Conversely, an exothermic reaction is. All phase changes are accompanied by changes in the energy of a system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). identifying exothermic & endothermic reactions. There are two methods for distinguishing between exothermic and endothermic reactions. the changes in energy that occur. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From printablekopierdu.z21.web.core.windows.net
Heat Of Phase Change How To Tell If A Phase Change Is Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. There are two methods for distinguishing between exothermic and endothermic reactions. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. identifying exothermic & endothermic reactions. . How To Tell If A Phase Change Is Endothermic Or Exothermic.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic How To Tell If A Phase Change Is Endothermic Or Exothermic identifying exothermic & endothermic reactions. changes of state are examples of phase changes, or phase transitions. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). All phase changes are accompanied by changes. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From schematicbosbokma.z4.web.core.windows.net
Phase Changes And Phase Diagrams How To Tell If A Phase Change Is Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. Conversely, an exothermic reaction is. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. identifying exothermic & endothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). All phase. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From lessonfullexcerption.z19.web.core.windows.net
Endothermic And Exothermic Explained How To Tell If A Phase Change Is Endothermic Or Exothermic There are two methods for distinguishing between exothermic and endothermic reactions. changes of state are examples of phase changes, or phase transitions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Conversely, an exothermic reaction is. identifying exothermic & endothermic reactions. an endothermic reaction occurs when energy is. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From www.diffzy.com
Exothermic vs. Endothermic Reaction What's the Difference (With Table) How To Tell If A Phase Change Is Endothermic Or Exothermic identifying exothermic & endothermic reactions. Conversely, an exothermic reaction is. There are two methods for distinguishing between exothermic and endothermic reactions. All phase changes are accompanied by changes in the energy of a system. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. the changes in energy that occur during. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From studylib.net
Endothermic vs. exothermic worksheet How To Tell If A Phase Change Is Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Conversely, an exothermic reaction is. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. an endothermic reaction. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From brainly.com
How to tell if a reaction is exothermic or endothermic from an equation How To Tell If A Phase Change Is Endothermic Or Exothermic the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. changes of state are examples of phase changes, or phase transitions. endothermic reactions are characterized by positive heat flow (into. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From inberp.info
Differences between exothermic and endothermic reactions (2023) How To Tell If A Phase Change Is Endothermic Or Exothermic There are two methods for distinguishing between exothermic and endothermic reactions. an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Conversely, an exothermic reaction is. endothermic reactions are characterized by. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From www.pinterest.com
Login to Your BenchPrep Account Teaching chemistry, Chemistry How To Tell If A Phase Change Is Endothermic Or Exothermic endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). There are two methods for distinguishing between exothermic and endothermic reactions. the changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. an endothermic reaction occurs when energy is absorbed from. How To Tell If A Phase Change Is Endothermic Or Exothermic.
From www.dreamstime.com
Exothermic and Endothermic Reactions in Chemistry Stock Illustration How To Tell If A Phase Change Is Endothermic Or Exothermic an endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). changes of state are examples of phase changes, or phase transitions. Conversely, an exothermic reaction is. All phase changes are accompanied by changes in. How To Tell If A Phase Change Is Endothermic Or Exothermic.