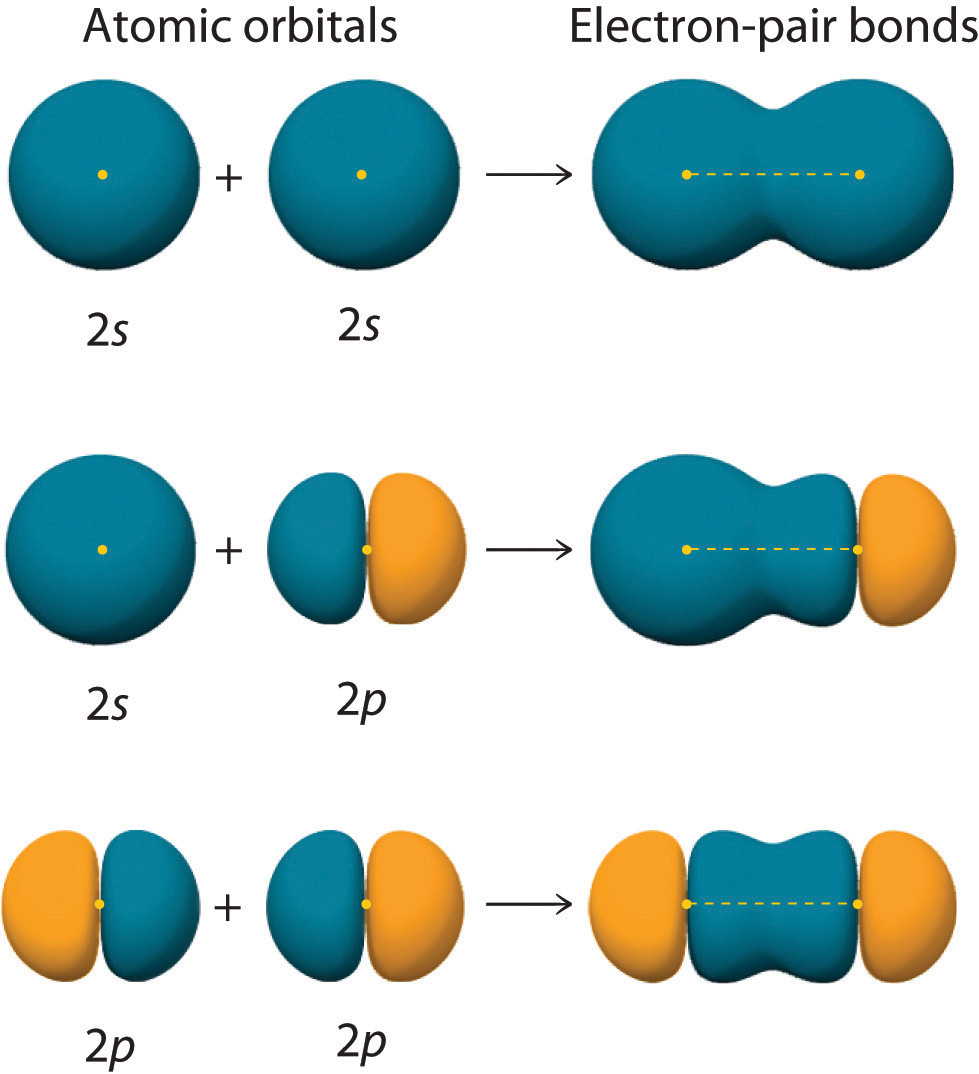
What Is A Bonding Orbital . bonding orbitals are overlapping molecular orbitals between two nuclei. Valence bond theory and molecular orbital. Electrons that spend most of their time between the nuclei of two atoms are placed into the. The two molecular orbitals of. we said in section 1.5 that chemists use two models for describing covalent bonds: An anti bonding orbital is located. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons?
from saylordotorg.github.io
the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. The two molecular orbitals of. An anti bonding orbital is located. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? bonding orbitals are overlapping molecular orbitals between two nuclei. Valence bond theory and molecular orbital. we said in section 1.5 that chemists use two models for describing covalent bonds: Electrons that spend most of their time between the nuclei of two atoms are placed into the.
Localized Bonding and Hybrid Atomic Orbitals
What Is A Bonding Orbital the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. we said in section 1.5 that chemists use two models for describing covalent bonds: The two molecular orbitals of. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. bonding orbitals are overlapping molecular orbitals between two nuclei. An anti bonding orbital is located. Valence bond theory and molecular orbital. Electrons that spend most of their time between the nuclei of two atoms are placed into the. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons?
From chem.libretexts.org
11.6 Delocalized Electrons Bonding in the Benzene Molecule What Is A Bonding Orbital An anti bonding orbital is located. we said in section 1.5 that chemists use two models for describing covalent bonds: in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? The two molecular orbitals of. bonding orbitals are overlapping molecular orbitals between two. What Is A Bonding Orbital.
From manuallibraryprehuman.z14.web.core.windows.net
Cn Molecular Orbital Diagram Bond Order What Is A Bonding Orbital An anti bonding orbital is located. Valence bond theory and molecular orbital. bonding orbitals are overlapping molecular orbitals between two nuclei. we said in section 1.5 that chemists use two models for describing covalent bonds: in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in. What Is A Bonding Orbital.
From www.coursehero.com
Bonding and Antibonding Molecular Orbitals Introduction to Chemistry What Is A Bonding Orbital bonding orbitals are overlapping molecular orbitals between two nuclei. we said in section 1.5 that chemists use two models for describing covalent bonds: Valence bond theory and molecular orbital. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. in what fundamental way does the molecular. What Is A Bonding Orbital.
From www.chemtube3d.com
SALC dd Orbital Overlap What Is A Bonding Orbital in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? we said in section 1.5 that chemists use two models for describing covalent bonds: Valence bond theory and molecular orbital. Electrons that spend most of their time between the nuclei of two atoms are. What Is A Bonding Orbital.
From mungfali.com
Sigma And Pi Bonds Examples What Is A Bonding Orbital we said in section 1.5 that chemists use two models for describing covalent bonds: The two molecular orbitals of. bonding orbitals are overlapping molecular orbitals between two nuclei. Electrons that spend most of their time between the nuclei of two atoms are placed into the. An anti bonding orbital is located. Valence bond theory and molecular orbital. . What Is A Bonding Orbital.
From wirepartdeclarator.z21.web.core.windows.net
Sigma And Pi Molecular Orbitals What Is A Bonding Orbital in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. The two molecular orbitals of. we said in section 1.5 that chemists use. What Is A Bonding Orbital.
From www.thoughtco.com
What Is an Antibonding Orbital? What Is A Bonding Orbital An anti bonding orbital is located. Electrons that spend most of their time between the nuclei of two atoms are placed into the. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. we said in section 1.5 that chemists use two models for describing covalent bonds: . What Is A Bonding Orbital.
From saylordotorg.github.io
Localized Bonding and Hybrid Atomic Orbitals What Is A Bonding Orbital An anti bonding orbital is located. we said in section 1.5 that chemists use two models for describing covalent bonds: in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? The two molecular orbitals of. bonding orbitals are overlapping molecular orbitals between two. What Is A Bonding Orbital.
From www.vrogue.co
Illustrated Glossary Of Organic Chemistry Sp2 Orbital vrogue.co What Is A Bonding Orbital The two molecular orbitals of. Electrons that spend most of their time between the nuclei of two atoms are placed into the. An anti bonding orbital is located. we said in section 1.5 that chemists use two models for describing covalent bonds: the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the. What Is A Bonding Orbital.
From www.myxxgirl.com
Bonding Theories Valence Bond Theory Molecular Orbital Theory My XXX What Is A Bonding Orbital in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? The two molecular orbitals of. we said in section 1.5 that chemists use two models for describing covalent bonds: Electrons that spend most of their time between the nuclei of two atoms are placed. What Is A Bonding Orbital.
From circuitenginegrave101.z5.web.core.windows.net
Ch2 Molecular Orbital Diagram What Is A Bonding Orbital Electrons that spend most of their time between the nuclei of two atoms are placed into the. we said in section 1.5 that chemists use two models for describing covalent bonds: bonding orbitals are overlapping molecular orbitals between two nuclei. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape. What Is A Bonding Orbital.
From enginedbtersanctus.z22.web.core.windows.net
Orbital Diagram For Be What Is A Bonding Orbital An anti bonding orbital is located. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. The two molecular orbitals of. bonding orbitals are overlapping molecular orbitals between two nuclei. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding. What Is A Bonding Orbital.
From schematicfixaiguille.z14.web.core.windows.net
2 Types Of Molecular Orbital Diagrams What Is A Bonding Orbital the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. we said in section 1.5 that chemists use two models for describing covalent bonds: Electrons that spend most of their time between the nuclei of two atoms are placed into the. in what fundamental way does the. What Is A Bonding Orbital.
From brilliant.org
chemical bonding molecular orbital theory Brilliant Math & Science Wiki What Is A Bonding Orbital Valence bond theory and molecular orbital. The two molecular orbitals of. bonding orbitals are overlapping molecular orbitals between two nuclei. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? An anti bonding orbital is located. the main difference between bonding and antibonding. What Is A Bonding Orbital.
From www.chemistrylibrary.org
Bonding and antibonding orbitals What Is A Bonding Orbital The two molecular orbitals of. Valence bond theory and molecular orbital. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? An anti bonding orbital is located. we said in section 1.5 that chemists use two models for describing covalent bonds: the main. What Is A Bonding Orbital.
From unacademy.com
Notes on Difference Between Bonding and Antibonding Molecular Orbitals What Is A Bonding Orbital the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. bonding orbitals are overlapping molecular orbitals between two nuclei. The two molecular orbitals of. An anti bonding orbital is located. we said in section 1.5 that chemists use two models for describing covalent bonds: Electrons that spend. What Is A Bonding Orbital.
From www.youtube.com
Hybrid Orbitals explained Valence Bond Theory Crash Chemistry What Is A Bonding Orbital in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. bonding orbitals are overlapping molecular orbitals between two nuclei. we said in. What Is A Bonding Orbital.
From geometrytipstekhnik.blogspot.com
37+ Molecular Orbital Geometry Image GM What Is A Bonding Orbital Electrons that spend most of their time between the nuclei of two atoms are placed into the. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. bonding orbitals are overlapping molecular orbitals between two nuclei. we said in section 1.5 that chemists use two models for. What Is A Bonding Orbital.
From pediaa.com
Difference Between Bonding and Antibonding Molecular Orbitals What Is A Bonding Orbital An anti bonding orbital is located. bonding orbitals are overlapping molecular orbitals between two nuclei. The two molecular orbitals of. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. we said in section 1.5 that chemists use two models for describing covalent bonds: Electrons that spend. What Is A Bonding Orbital.
From classnotes.org.in
Types of Molecular Orbital Formed Chemical Bonding and Molecular What Is A Bonding Orbital Electrons that spend most of their time between the nuclei of two atoms are placed into the. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? The two molecular orbitals of. An anti bonding orbital is located. we said in section 1.5 that. What Is A Bonding Orbital.
From chem.libretexts.org
Chapter 6.5 Delocalized Bonding and Molecular Orbitals Chemistry What Is A Bonding Orbital in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? The two molecular orbitals of. bonding orbitals are overlapping molecular orbitals between two nuclei. Valence bond theory and molecular orbital. the main difference between bonding and antibonding molecular orbitals is that bonding molecular. What Is A Bonding Orbital.
From mavink.com
Bonding Vs Antibonding Orbitals What Is A Bonding Orbital The two molecular orbitals of. An anti bonding orbital is located. Valence bond theory and molecular orbital. Electrons that spend most of their time between the nuclei of two atoms are placed into the. bonding orbitals are overlapping molecular orbitals between two nuclei. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent. What Is A Bonding Orbital.
From www.youtube.com
CHEMISTRY 101 Molecular Orbital Theory, Bond order, bond strength What Is A Bonding Orbital Valence bond theory and molecular orbital. The two molecular orbitals of. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. we said in section 1.5 that chemists use two models for describing covalent bonds: in what fundamental way does the molecular orbital model differ from the. What Is A Bonding Orbital.
From madoverchemistry.wordpress.com
89. CHEMICAL BONDING (36) Covalent Bonding(35) Molecular Orbital What Is A Bonding Orbital The two molecular orbitals of. we said in section 1.5 that chemists use two models for describing covalent bonds: An anti bonding orbital is located. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. Electrons that spend most of their time between the nuclei of two atoms. What Is A Bonding Orbital.
From mydiagram.online
[DIAGRAM] Single Phase Bonding Diagram What Is A Bonding Orbital we said in section 1.5 that chemists use two models for describing covalent bonds: Electrons that spend most of their time between the nuclei of two atoms are placed into the. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? Valence bond theory. What Is A Bonding Orbital.
From circuitgestrinovu6.z21.web.core.windows.net
How To Draw Molecular Orbital Diagrams What Is A Bonding Orbital bonding orbitals are overlapping molecular orbitals between two nuclei. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. Electrons that spend most of their time between the nuclei of two atoms are placed into the. we said in section 1.5 that chemists use two models for. What Is A Bonding Orbital.
From brilliant.org
Molecular Orbital Theory Brilliant Math & Science Wiki What Is A Bonding Orbital Valence bond theory and molecular orbital. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? The two molecular orbitals of. An anti bonding orbital is located. bonding orbitals are overlapping molecular orbitals between two nuclei. Electrons that spend most of their time between. What Is A Bonding Orbital.
From www.masterorganicchemistry.com
Bonding And Antibonding Pi Orbitals Master Organic Chemistry What Is A Bonding Orbital The two molecular orbitals of. An anti bonding orbital is located. Valence bond theory and molecular orbital. Electrons that spend most of their time between the nuclei of two atoms are placed into the. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. bonding orbitals are overlapping. What Is A Bonding Orbital.
From pressbooks.online.ucf.edu
7.7 Molecular Orbital Theory Chemistry Fundamentals What Is A Bonding Orbital the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? The two molecular orbitals of. we said in section 1.5 that chemists use. What Is A Bonding Orbital.
From www.animalia-life.club
Atomic Orbitals Vs Molecular Orbitals What Is A Bonding Orbital Electrons that spend most of their time between the nuclei of two atoms are placed into the. The two molecular orbitals of. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. Valence bond theory and molecular orbital. in what fundamental way does the molecular orbital model differ. What Is A Bonding Orbital.
From socratic.org
What is the difference between bonding and antibonding molecular What Is A Bonding Orbital Electrons that spend most of their time between the nuclei of two atoms are placed into the. Valence bond theory and molecular orbital. An anti bonding orbital is located. The two molecular orbitals of. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. in what fundamental way. What Is A Bonding Orbital.
From favpng.com
Atomic Orbital Molecular Orbital Diagram Chemical Bond Quintuple Bond What Is A Bonding Orbital Electrons that spend most of their time between the nuclei of two atoms are placed into the. An anti bonding orbital is located. The two molecular orbitals of. Valence bond theory and molecular orbital. we said in section 1.5 that chemists use two models for describing covalent bonds: bonding orbitals are overlapping molecular orbitals between two nuclei. . What Is A Bonding Orbital.
From www.britannica.com
Coordination compound Ligand Field, MO Theory Britannica What Is A Bonding Orbital in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? The two molecular orbitals of. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. we said in section 1.5 that chemists use. What Is A Bonding Orbital.
From byjus.com
A π bond is formed by the overlap of What Is A Bonding Orbital An anti bonding orbital is located. the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals represent the shape of a molecule. Valence bond theory and molecular orbital. in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? bonding. What Is A Bonding Orbital.
From quizlet.com
Draw the molecular orbital diagram of \ce{Be2}. Quizlet What Is A Bonding Orbital The two molecular orbitals of. we said in section 1.5 that chemists use two models for describing covalent bonds: in what fundamental way does the molecular orbital model differ from the other models of chemical bonding that have been described in these lessons? the main difference between bonding and antibonding molecular orbitals is that bonding molecular orbitals. What Is A Bonding Orbital.