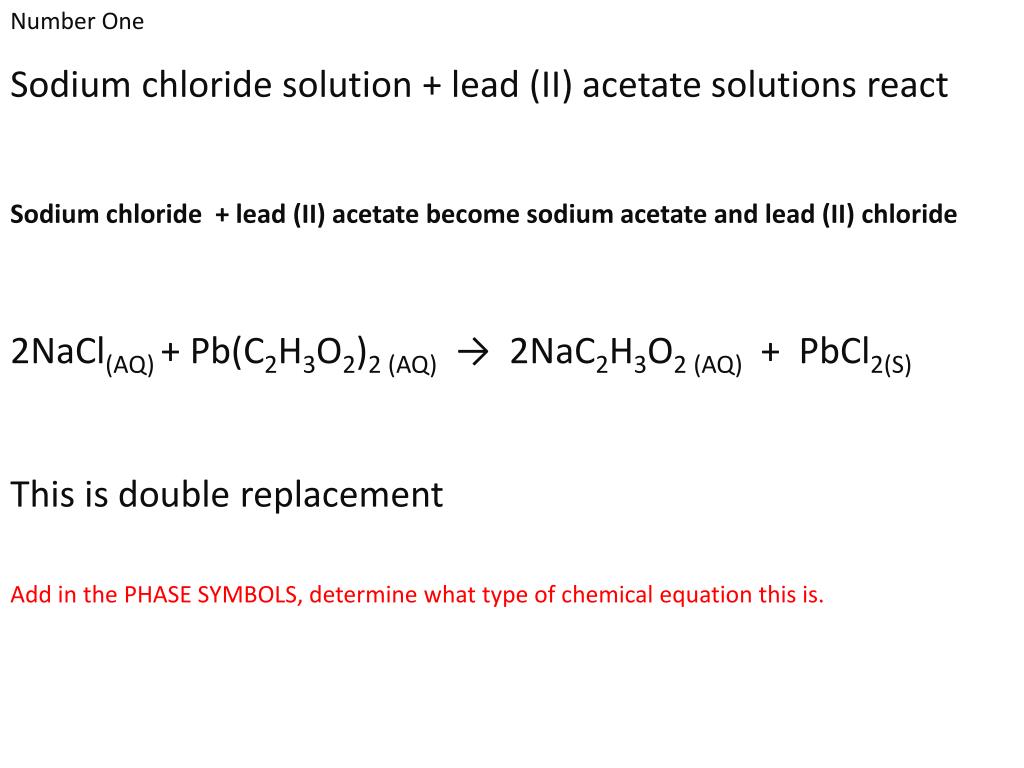
Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation . To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. When sodium chloride is added to the lead nitrate solution a precipitate of lead. Reaction between sodium chloride and lead nitrate solution: Enter an equation of an ionic chemical equation and press the balance button. Write a balanced molecular and net ionic equation for. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. The balanced equation will be calculated along with the. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. But for the net ionic equation, we represent only the net, macroscopic chemical change:
from exoncvmev.blob.core.windows.net
The balanced equation will appear. Write a balanced molecular and net ionic equation for. But for the net ionic equation, we represent only the net, macroscopic chemical change: The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. When sodium chloride is added to the lead nitrate solution a precipitate of lead. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Reaction between sodium chloride and lead nitrate solution: The balanced equation will be calculated along with the.
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons
Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Reaction between sodium chloride and lead nitrate solution: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will be calculated along with the. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The balanced equation will appear. Reaction between sodium chloride and lead nitrate solution: When sodium chloride is added to the lead nitrate solution a precipitate of lead. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. But for the net ionic equation, we represent only the net, macroscopic chemical change: Enter an equation of an ionic chemical equation and press the balance button. Write a balanced molecular and net ionic equation for.
From www.chegg.com
Solved Predict products, and write balanced net ionic Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Reaction between sodium chloride and lead nitrate solution: Write a balanced molecular and net ionic equation for. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The balanced equation will appear. But. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Reaction between sodium chloride and lead nitrate solution: The balanced equation will appear. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Write a balanced molecular and net ionic equation for. When sodium chloride is added to the lead nitrate solution a precipitate of lead. The balanced equation will. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.chegg.com
Solved Lead (II) nitrate reacts with sodium iodide to Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Write a balanced molecular and net ionic equation for. But for the net ionic equation, we represent only the net, macroscopic chemical change: Enter an equation of an ionic chemical equation and press the balance button. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The products of the reaction are an. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.slideserve.com
PPT Reactions Involving Ions Molecular vs. Ionic Equations Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation But for the net ionic equation, we represent only the net, macroscopic chemical change: The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The balanced equation will appear. The balanced equation will be calculated along with the. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate.. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation When sodium chloride is added to the lead nitrate solution a precipitate of lead. The balanced equation will be calculated along with the. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. Enter an equation of an. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.youtube.com
Write the balanced chemical equation for each of the following Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Reaction between sodium chloride and lead nitrate solution: Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. But for the net ionic equation, we represent only the net, macroscopic chemical change: When sodium chloride is added to the lead nitrate. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From picklifestyles.blogspot.com
Lead II Nitrate Reaction With Potassium Iodide Pb(NO3)2 Lifestyle News Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. When sodium chloride is added to the lead nitrate solution a precipitate of lead. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.slideserve.com
PPT Precipitation Reactions (Double Replacement Reactions) PowerPoint Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation When sodium chloride is added to the lead nitrate solution a precipitate of lead. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will be calculated along with the. Write a balanced molecular and net ionic equation. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.coursehero.com
[Solved] Lead(II) nitrate reacts with potassium chromate to form lead Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Write a balanced molecular and net ionic equation for. The balanced equation will appear. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Lead(ii) nitrate reacts with sodium chloride. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Sodium Chloride Tessshebaylo Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation When sodium chloride is added to the lead nitrate solution a precipitate of lead. Reaction between sodium chloride and lead nitrate solution: Write a balanced molecular and net ionic equation for. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Lead(ii) nitrate reacts with sodium chloride in. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From askfilo.com
I Reactions and Equations Silver nitrate + Sodium chloride Silver chlor.. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation When sodium chloride is added to the lead nitrate solution a precipitate of lead. Reaction between sodium chloride and lead nitrate solution: Write a balanced molecular and net ionic equation for. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. Enter an equation of an ionic chemical equation and press the balance button. The products of the. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead (ii) chloride Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Write a balanced molecular and net ionic equation for. Reaction between sodium chloride and lead nitrate solution: But for the net ionic equation, we represent only the net, macroscopic chemical change: The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. When sodium chloride is added to the lead nitrate. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.chegg.com
Solved If 1 mole of lead (II) nitrate reacts with 1 mole of Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Reaction between sodium chloride and lead nitrate solution: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The products of the reaction are an aqueous solution of sodium nitrate and a solid. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Write a balanced molecular and net ionic equation for. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. When sodium chloride is added to the lead nitrate solution a precipitate of lead. Reaction between. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From elchoroukhost.net
Balanced Chemical Equation For Table Salt And Silver Nitrate Elcho Table Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation The balanced equation will appear. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write a balanced molecular and net ionic equation for. But for the net ionic equation, we represent only the net, macroscopic chemical change: The products of. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.chegg.com
Solved When Copper (II) Chloride Reacts With Sodium Nitra... Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Reaction between sodium chloride and lead nitrate solution: The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.youtube.com
Silver nitrate + sodium chloride YouTube Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The balanced equation will appear. Reaction between sodium chloride and lead nitrate solution: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The products of the reaction are an aqueous solution of. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Reaction between sodium chloride and lead nitrate solution: The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Enter an equation of an ionic chemical equation and press the balance button. The balanced. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.youtube.com
Double displacement NaCl + Pb(NO3)2 Sodium chloride + Lead(ii Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Reaction between sodium chloride and lead nitrate solution: The balanced equation will appear. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. Write a balanced molecular and net ionic equation for. The products of the reaction are. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Reaction between sodium chloride and lead nitrate solution: The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Enter an equation of an ionic chemical equation and press the balance button. When sodium chloride is added to the lead nitrate solution a precipitate of lead. Lead(ii) nitrate reacts with sodium. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.chegg.com
Solved When sodium chloride and lead (II) nitrate react in Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. Reaction between sodium chloride and lead nitrate solution: The balanced equation will be calculated along with the. Write a balanced molecular and net ionic equation for.. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.coursehero.com
[Solved] 13_ Aqueous lead (1]) nitrate, Ph[N03)2 undergoes a double Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Reaction between sodium chloride and lead nitrate solution: Write a balanced molecular and net ionic equation for. The balanced equation will appear. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.coursehero.com
[Solved] Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. When sodium chloride is added to the. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.coursehero.com
[Solved] If I start with 25.0 grams of lead (II) nitrate and 15.0 grams Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Write a balanced molecular and net ionic equation for. Enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. But for the net ionic equation, we represent only the net, macroscopic chemical change: The balanced equation will. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation The balanced equation will appear. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Reaction between sodium chloride and lead nitrate solution: The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. To balance a chemical equation, enter an. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. But for the net ionic equation, we represent only the net, macroscopic chemical change: Enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of sodium. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From hxemtsrvi.blob.core.windows.net
Lead Ii Nitrate Equation at Donald Ramos blog Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Write a balanced molecular and net ionic equation for. The balanced equation will appear. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. When sodium chloride is added to the lead nitrate. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.numerade.com
SOLVEDIf 96.3 mL of lead(II) nitrate solution reacts completely with Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. But for the net ionic equation, we represent only the net, macroscopic chemical change: Lead(ii) nitrate. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Write a balanced molecular and net ionic equation for. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will appear. When sodium chloride is added to the lead nitrate solution a precipitate of. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Write a balanced molecular and net ionic equation. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Reaction between sodium chloride and lead nitrate solution: Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. The balanced equation will be calculated along with the. Write a balanced molecular and net ionic equation for. Enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Reaction between sodium chloride and lead nitrate solution: But for the net ionic equation, we represent only the net, macroscopic. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From wisc.pb.unizin.org
Solutions and Solubility (part 2) (M3Q2) UWMadison Chemistry 103/104 Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. Write a balanced molecular and net ionic equation for. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. But for the net ionic equation, we represent only the net, macroscopic chemical change: When sodium chloride is added to the lead. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From www.numerade.com
SOLVEDWhen a solution of lead(II) nitrate is mixed with a solution of Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation The balanced equation will be calculated along with the. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Lead(ii) nitrate reacts with sodium chloride in aqueous solution to form precipitate. But for the net ionic equation, we represent only the net, macroscopic chemical change: When sodium chloride is added. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.
From tiannagroharvey.blogspot.com
Aqueous Sodium Chloride Reacts With Aqueous Lead Ii Nitrate Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation Write a balanced molecular and net ionic equation for. But for the net ionic equation, we represent only the net, macroscopic chemical change: The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (ii) chloride. Reaction between sodium chloride and lead nitrate solution: When sodium chloride is added to the lead nitrate. Lead Ii Nitrate Reacts With Sodium Chloride Balanced Equation.