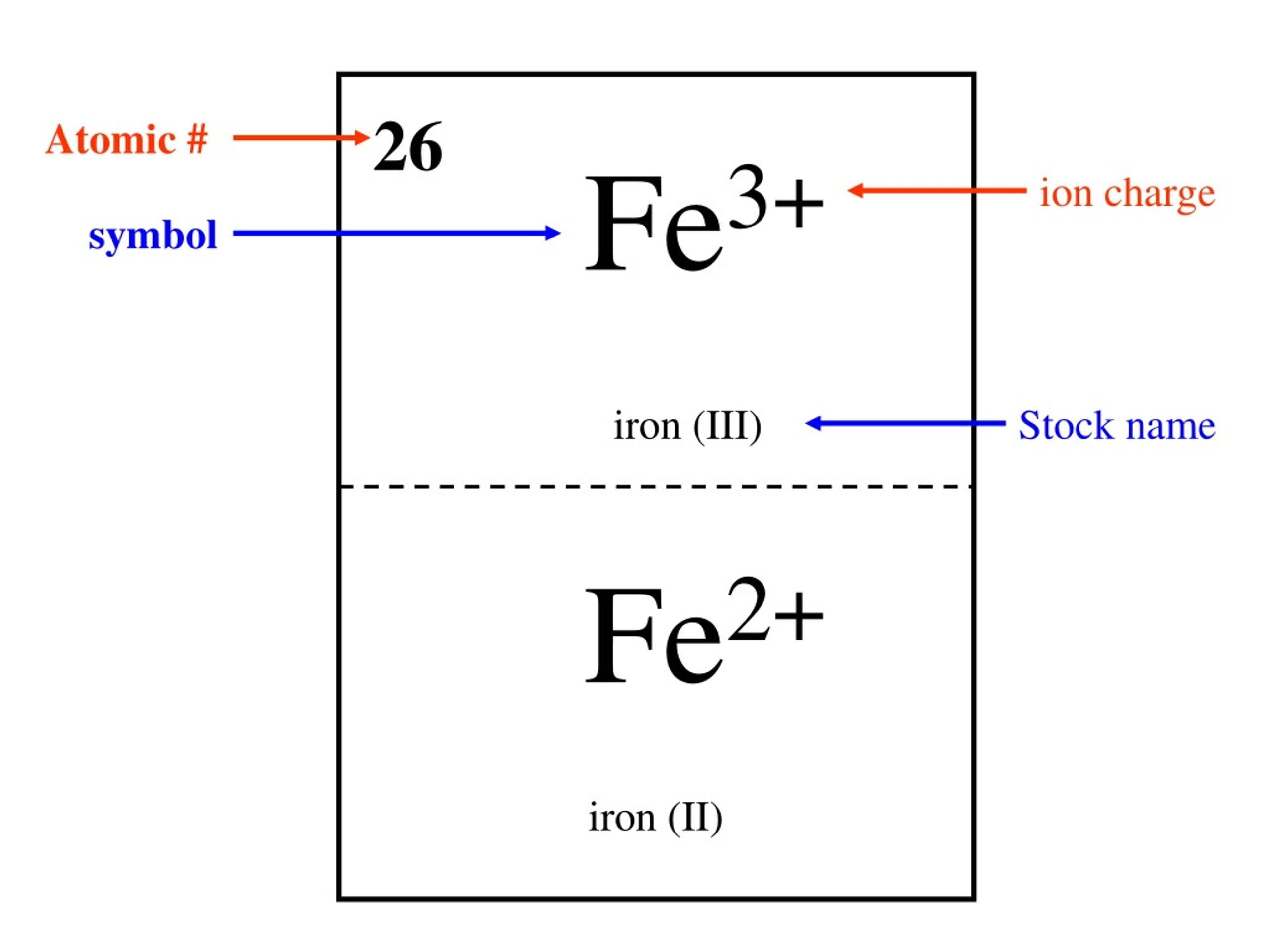
Which Iron Charge Is More Common . This electric charge generated on the ion is. The common ionic charges of iron (fe) are 2+ and 3+. When the atom loses electron/s, it forms positively charged ion. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. 93 rows ionic charge: This particular can have different numbers of electrons but we’ll say this example has 23 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Looking for a periodic table of elements with common charges? But the question is how can you find the ionic charge on iron (fe)? An atom of iron (fe) has 26 protons. This table contains the most common charges along with.
from www.slideserve.com
This electric charge generated on the ion is. Looking for a periodic table of elements with common charges? This particular can have different numbers of electrons but we’ll say this example has 23 electrons. But the question is how can you find the ionic charge on iron (fe)? The common ionic charges of iron (fe) are 2+ and 3+. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. An atom of iron (fe) has 26 protons. 93 rows ionic charge: For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. When the atom loses electron/s, it forms positively charged ion.
PPT Chemical Nomenclature (Naming Compounds) PowerPoint Presentation
Which Iron Charge Is More Common An atom of iron (fe) has 26 protons. An atom of iron (fe) has 26 protons. The common ionic charges of iron (fe) are 2+ and 3+. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). But the question is how can you find the ionic charge on iron (fe)? Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. This table contains the most common charges along with. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. When the atom loses electron/s, it forms positively charged ion. Looking for a periodic table of elements with common charges? This electric charge generated on the ion is. 93 rows ionic charge: For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state.
From www.printabletemplate.us
Periodic Table Printable With Charges Which Iron Charge Is More Common Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. When the atom loses electron/s, it forms positively charged ion. This electric charge generated on the ion is. The common ionic charges of iron (fe) are 2+ and 3+. But the question is how can you find the ionic charge on iron. Which Iron Charge Is More Common.
From awesomehome.co
Iron Periodic Table Charge Awesome Home Which Iron Charge Is More Common For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. The common ionic charges of iron (fe) are 2+ and 3+. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: This electric charge generated on. Which Iron Charge Is More Common.
From periodictable.me
How Many Valence Electrons Does Iron have Archives Dynamic Periodic Which Iron Charge Is More Common The common ionic charges of iron (fe) are 2+ and 3+. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. But the question is how can you find the ionic charge on iron (fe)? When the atom loses electron/s, it forms positively charged ion. When the atom loses or. Which Iron Charge Is More Common.
From www.deviantart.com
0737 Iron Charge by MiNorlax on DeviantArt Which Iron Charge Is More Common When the atom loses electron/s, it forms positively charged ion. An atom of iron (fe) has 26 protons. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. But the question is how can you find the ionic charge on iron (fe)? This table contains the most common charges along. Which Iron Charge Is More Common.
From gionieccf.blob.core.windows.net
Iron Charge Of 2 at Katherine Romero blog Which Iron Charge Is More Common An atom of iron (fe) has 26 protons. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Ionic charge is the electrical charge that is generated on the atom. Which Iron Charge Is More Common.
From www.sliderbase.com
Ionic Compound Nomenclature Presentation Chemistry Which Iron Charge Is More Common The common ionic charges of iron (fe) are 2+ and 3+. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When the atom loses electron/s, it forms positively charged ion. This. Which Iron Charge Is More Common.
From www.researchgate.net
Snapshot of the average iron charge state Q Fe spatial distribution Which Iron Charge Is More Common 93 rows ionic charge: This table contains the most common charges along with. An atom of iron (fe) has 26 protons. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. The common ionic charges of iron (fe) are 2+ and 3+. When the atom loses electron/s, it forms positively charged ion.. Which Iron Charge Is More Common.
From aznswerzoneticbacchanals.z21.web.core.windows.net
How To Find Polyatomic Ion Formulas Which Iron Charge Is More Common For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. This table contains the most common charges along with. When the atom loses electron/s, it forms positively charged ion. But the question. Which Iron Charge Is More Common.
From www.flinnsci.ca
Ion Names, Formulas and Charges Chart Flinn Scientific Which Iron Charge Is More Common For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. When the atom loses electron/s, it forms positively charged ion. Looking for a periodic table of elements with common charges? This electric charge generated. Which Iron Charge Is More Common.
From asyikinkimia7.blogspot.com
BETTER LIVING THROUGH CHEMISTRY Common polyatomic ions Which Iron Charge Is More Common This electric charge generated on the ion is. Looking for a periodic table of elements with common charges? 93 rows ionic charge: The common ionic charges of iron (fe) are 2+ and 3+. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. For example, copper usually has a +1 or +2 valence, while. Which Iron Charge Is More Common.
From www.artstation.com
ArtStation Iron Charge Which Iron Charge Is More Common This particular can have different numbers of electrons but we’ll say this example has 23 electrons. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. 93 rows ionic charge: This table contains the most common charges along with. When the atom loses electron/s, it forms positively charged ion. This. Which Iron Charge Is More Common.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Which Iron Charge Is More Common An atom of iron (fe) has 26 protons. When the atom loses electron/s, it forms positively charged ion. The common ionic charges of iron (fe) are 2+ and 3+. This table contains the most common charges along with. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. When the atom loses. Which Iron Charge Is More Common.
From elchoroukhost.net
Periodic Table With Ionic Charges And Names Elcho Table Which Iron Charge Is More Common But the question is how can you find the ionic charge on iron (fe)? Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. An atom of iron (fe) has 26 protons. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state.. Which Iron Charge Is More Common.
From www.semanticscholar.org
Figure 3 from Iron Charge Distribution as an Identifier of Which Iron Charge Is More Common Looking for a periodic table of elements with common charges? This table contains the most common charges along with. The common ionic charges of iron (fe) are 2+ and 3+. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. But the question is how can you find the ionic charge on. Which Iron Charge Is More Common.
From www.nigerianeye.com
Why Young Women Are More Prone to Iron Deficiency Nigerian News Which Iron Charge Is More Common Looking for a periodic table of elements with common charges? An atom of iron (fe) has 26 protons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). But the question is how can you find the ionic charge on iron (fe)? This electric charge generated on the ion is.. Which Iron Charge Is More Common.
From giomcvzog.blob.core.windows.net
Iron Negative Charge at Sherman Anthony blog Which Iron Charge Is More Common This particular can have different numbers of electrons but we’ll say this example has 23 electrons. But the question is how can you find the ionic charge on iron (fe)? When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The common ionic charges of iron (fe) are 2+ and. Which Iron Charge Is More Common.
From gioagjpux.blob.core.windows.net
Acrylic Vs Cast Iron Freestanding Tub at Marie Adams blog Which Iron Charge Is More Common 93 rows ionic charge: This electric charge generated on the ion is. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. This table contains the most common charges along with. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Looking for a. Which Iron Charge Is More Common.
From gioagjpux.blob.core.windows.net
Acrylic Vs Cast Iron Freestanding Tub at Marie Adams blog Which Iron Charge Is More Common When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Looking for a periodic table of elements with common charges? Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. An atom of iron (fe) has 26 protons. This table contains the. Which Iron Charge Is More Common.
From giomcvzog.blob.core.windows.net
Iron Negative Charge at Sherman Anthony blog Which Iron Charge Is More Common The common ionic charges of iron (fe) are 2+ and 3+. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. This electric charge generated on the ion is. An atom of iron (fe) has 26 protons. 93 rows ionic charge: When the atom loses electron/s, it forms positively charged ion. This table contains. Which Iron Charge Is More Common.
From alquilercastilloshinchables.info
8 Photos Iron Periodic Table And Description Alqu Blog Which Iron Charge Is More Common Looking for a periodic table of elements with common charges? The common ionic charges of iron (fe) are 2+ and 3+. This table contains the most common charges along with. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. But the question is how can you find the ionic charge on iron (fe)?. Which Iron Charge Is More Common.
From giossqlsz.blob.core.windows.net
Iron Powder Charge at Mike Thaxton blog Which Iron Charge Is More Common An atom of iron (fe) has 26 protons. Looking for a periodic table of elements with common charges? This particular can have different numbers of electrons but we’ll say this example has 23 electrons. This electric charge generated on the ion is. When the atom loses electron/s, it forms positively charged ion. 93 rows ionic charge: The common ionic charges. Which Iron Charge Is More Common.
From giogcfjnd.blob.core.windows.net
Iron Definition Longman at Brian Sullivan blog Which Iron Charge Is More Common Looking for a periodic table of elements with common charges? This table contains the most common charges along with. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. When the atom loses electron/s, it forms positively charged ion. This electric charge generated on the ion is. Ionic charge is. Which Iron Charge Is More Common.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Which Iron Charge Is More Common For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. This electric charge generated on the ion is. When the atom loses electron/s, it forms positively charged ion. Ionic charge is the electrical charge. Which Iron Charge Is More Common.
From www.researchgate.net
Iron charge state vs. day of year ( DOY) in 2002 from the Ulysses Which Iron Charge Is More Common An atom of iron (fe) has 26 protons. This table contains the most common charges along with. But the question is how can you find the ionic charge on iron (fe)? This electric charge generated on the ion is. Looking for a periodic table of elements with common charges? When the atom loses or gains one or more electrons, the. Which Iron Charge Is More Common.
From ar.inspiredpencil.com
Periodic Table With Charges And Polyatomic Ions Which Iron Charge Is More Common The common ionic charges of iron (fe) are 2+ and 3+. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. Looking for a periodic table of elements with common charges? When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. Which Iron Charge Is More Common.
From giomcvzog.blob.core.windows.net
Iron Negative Charge at Sherman Anthony blog Which Iron Charge Is More Common Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. An atom of iron (fe) has 26 protons. When the atom loses electron/s, it forms positively charged ion. This table contains the most common charges along with. This electric charge generated on the ion is. Looking for a periodic table of elements. Which Iron Charge Is More Common.
From www.slideserve.com
PPT Chemical Nomenclature (Naming Compounds) PowerPoint Presentation Which Iron Charge Is More Common For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. The common ionic charges of iron (fe) are 2+ and 3+. When the atom loses or gains one or more electrons, the. Which Iron Charge Is More Common.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom Which Iron Charge Is More Common 93 rows ionic charge: This electric charge generated on the ion is. Looking for a periodic table of elements with common charges? When the atom loses electron/s, it forms positively charged ion. This table contains the most common charges along with. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation. Which Iron Charge Is More Common.
From publicaffairsworld.com
what is irons charge Which Iron Charge Is More Common But the question is how can you find the ionic charge on iron (fe)? For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. This electric charge generated on the ion is. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. When the. Which Iron Charge Is More Common.
From giomcvzog.blob.core.windows.net
Iron Negative Charge at Sherman Anthony blog Which Iron Charge Is More Common 93 rows ionic charge: An atom of iron (fe) has 26 protons. The common ionic charges of iron (fe) are 2+ and 3+. This table contains the most common charges along with. This electric charge generated on the ion is. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state.. Which Iron Charge Is More Common.
From www.compoundchem.com
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest Which Iron Charge Is More Common Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. For example, copper usually has a +1 or +2 valence, while iron typically has a +2 or +3 oxidation state. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When the. Which Iron Charge Is More Common.
From gionieccf.blob.core.windows.net
Iron Charge Of 2 at Katherine Romero blog Which Iron Charge Is More Common Looking for a periodic table of elements with common charges? The common ionic charges of iron (fe) are 2+ and 3+. But the question is how can you find the ionic charge on iron (fe)? An atom of iron (fe) has 26 protons. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. Ionic. Which Iron Charge Is More Common.
From bigpivots.com
Ironair batteries to displace natural gas in firming the grid? Big Which Iron Charge Is More Common 93 rows ionic charge: But the question is how can you find the ionic charge on iron (fe)? Looking for a periodic table of elements with common charges? When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When the atom loses electron/s, it forms positively charged ion. For example,. Which Iron Charge Is More Common.
From vancouverdas.weebly.com
Simple printable periodic table of elements with charges vancouverdas Which Iron Charge Is More Common 93 rows ionic charge: When the atom loses electron/s, it forms positively charged ion. This electric charge generated on the ion is. The common ionic charges of iron (fe) are 2+ and 3+. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). An atom of iron (fe) has 26. Which Iron Charge Is More Common.
From brandonkss.github.io
Cations And Anions Chart Which Iron Charge Is More Common Looking for a periodic table of elements with common charges? This electric charge generated on the ion is. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. An atom of iron (fe) has 26 protons. The common ionic charges of iron (fe) are 2+ and 3+. Ionic charge is the electrical charge that. Which Iron Charge Is More Common.