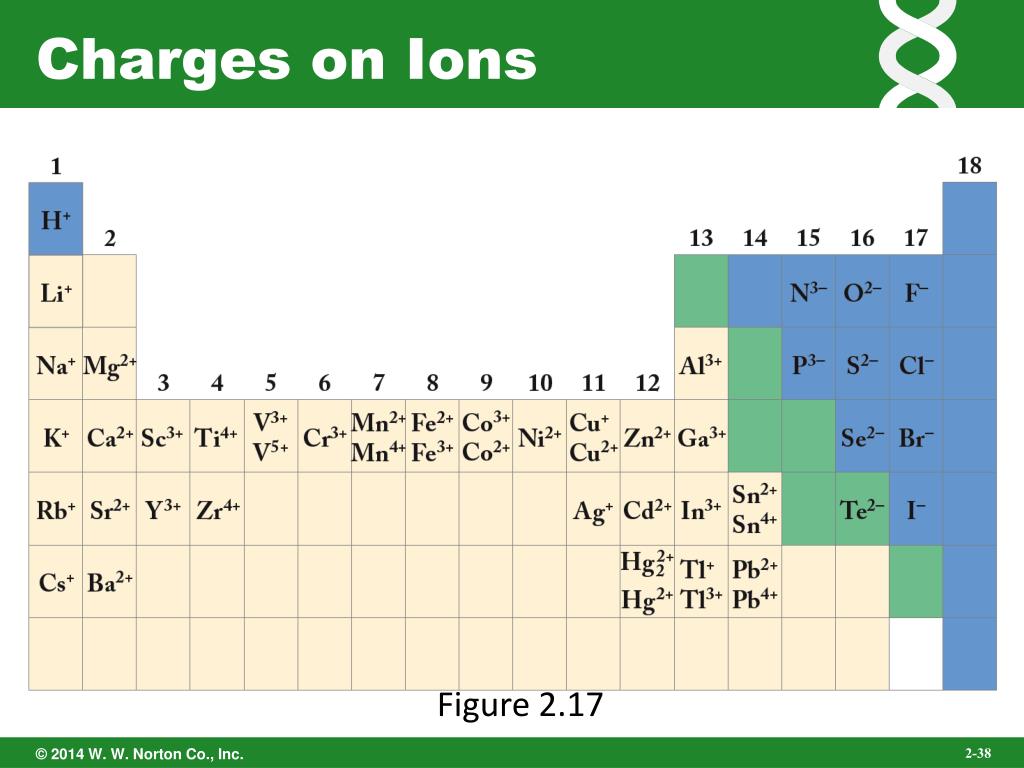
Aluminum Charges . 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. using a simple, general trend for the ionic charge for elements on the. aluminium has a charge of +3. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. Aluminium has 13 electrons and 13 protons. This is because the element’s atomic number is 13, reflecting the fact. the charge of an aluminum ion is typically 3+. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. All group 17 elements (halogens) gain one. These electrons are distributed across the shells in the.
from
during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. Aluminium has 13 electrons and 13 protons. using a simple, general trend for the ionic charge for elements on the. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. aluminium has a charge of +3. This is because the element’s atomic number is 13, reflecting the fact. the charge of an aluminum ion is typically 3+. These electrons are distributed across the shells in the. All group 17 elements (halogens) gain one. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge.
Aluminum Charges Aluminium has 13 electrons and 13 protons. using a simple, general trend for the ionic charge for elements on the. This is because the element’s atomic number is 13, reflecting the fact. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. Aluminium has 13 electrons and 13 protons. All group 17 elements (halogens) gain one. the charge of an aluminum ion is typically 3+. These electrons are distributed across the shells in the. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. aluminium has a charge of +3.
From
Aluminum Charges group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. This is because the element’s atomic number is 13, reflecting the fact. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. All group 17 elements (halogens) gain one. the charge of an aluminum ion is. Aluminum Charges.
From www.britannica.com
aluminum Uses, Properties, & Compounds Britannica Aluminum Charges Aluminium has 13 electrons and 13 protons. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. aluminium has a charge of +3. the charge of an aluminum ion is typically 3+. This is because. Aluminum Charges.
From
Aluminum Charges using a simple, general trend for the ionic charge for elements on the. Aluminium has 13 electrons and 13 protons. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group. Aluminum Charges.
From
Aluminum Charges This is because the element’s atomic number is 13, reflecting the fact. These electrons are distributed across the shells in the. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. the charge of an aluminum ion is typically 3+. group 13 metals like aluminum lose three electrons to form an ion. Aluminum Charges.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom Aluminum Charges These electrons are distributed across the shells in the. This is because the element’s atomic number is 13, reflecting the fact. aluminium has a charge of +3. using a simple, general trend for the ionic charge for elements on the. the charge of an aluminum ion is typically 3+. during the chemical reaction, aluminum loses these. Aluminum Charges.
From
Aluminum Charges using a simple, general trend for the ionic charge for elements on the. Aluminium has 13 electrons and 13 protons. aluminium has a charge of +3. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. This is because the element’s atomic number is 13, reflecting the fact. 93 rows. Aluminum Charges.
From thechemistrynotes.com
Aluminium (Al) Element Important Information, Properties, Uses Aluminum Charges the charge of an aluminum ion is typically 3+. aluminium has a charge of +3. Aluminium has 13 electrons and 13 protons. These electrons are distributed across the shells in the. using a simple, general trend for the ionic charge for elements on the. This is because the element’s atomic number is 13, reflecting the fact. . Aluminum Charges.
From
Aluminum Charges This is because the element’s atomic number is 13, reflecting the fact. the charge of an aluminum ion is typically 3+. Aluminium has 13 electrons and 13 protons. aluminium has a charge of +3. These electrons are distributed across the shells in the. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble. Aluminum Charges.
From
Aluminum Charges aluminium has a charge of +3. the charge of an aluminum ion is typically 3+. These electrons are distributed across the shells in the. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. Aluminium has 13 electrons and 13 protons. 93 rows for example, group i elements on the. Aluminum Charges.
From chemistry291.blogspot.com
What Is the Chemical Formula for Aluminum Oxide? Aluminum Charges during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. aluminium has a charge of +3. This is because the element’s atomic number is 13, reflecting. Aluminum Charges.
From cartoondealer.com
Aluminium Aluminum Metal, Crystal Structure. RoyaltyFree Stock Photo Aluminum Charges aluminium has a charge of +3. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. This is because the element’s atomic number is 13, reflecting the fact. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. 93 rows for example, group i elements. Aluminum Charges.
From
Aluminum Charges during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. These electrons are distributed across the shells in the. All group 17 elements (halogens) gain one. the charge of an aluminum ion is typically 3+. Aluminium has 13 electrons and 13 protons. 93 rows for example, group i elements on the periodic. Aluminum Charges.
From brainly.in
Atomic structure of aluminum Brainly.in Aluminum Charges aluminium has a charge of +3. the charge of an aluminum ion is typically 3+. This is because the element’s atomic number is 13, reflecting the fact. Aluminium has 13 electrons and 13 protons. These electrons are distributed across the shells in the. group 13 metals like aluminum lose three electrons to form an ion with a. Aluminum Charges.
From
Aluminum Charges Aluminium has 13 electrons and 13 protons. This is because the element’s atomic number is 13, reflecting the fact. aluminium has a charge of +3. using a simple, general trend for the ionic charge for elements on the. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. 93 rows. Aluminum Charges.
From
Aluminum Charges the charge of an aluminum ion is typically 3+. using a simple, general trend for the ionic charge for elements on the. aluminium has a charge of +3. This is because the element’s atomic number is 13, reflecting the fact. All group 17 elements (halogens) gain one. during the chemical reaction, aluminum loses these 3 electrons. Aluminum Charges.
From
Aluminum Charges using a simple, general trend for the ionic charge for elements on the. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. These electrons are distributed across the shells in the. All group 17 elements. Aluminum Charges.
From
Aluminum Charges These electrons are distributed across the shells in the. aluminium has a charge of +3. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. the charge of an aluminum ion is typically 3+. This is because the element’s atomic number is 13, reflecting the fact. Aluminium has 13 electrons and 13. Aluminum Charges.
From
Aluminum Charges using a simple, general trend for the ionic charge for elements on the. aluminium has a charge of +3. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. All group 17 elements (halogens) gain one. group 13 metals like. Aluminum Charges.
From tmpartnersgroup.com
How Is Aluminum Made? TM Partners Aluminum Charges the charge of an aluminum ion is typically 3+. All group 17 elements (halogens) gain one. These electrons are distributed across the shells in the. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. group 13 metals like aluminum lose. Aluminum Charges.
From
Aluminum Charges These electrons are distributed across the shells in the. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. All group 17 elements (halogens) gain one. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as.. Aluminum Charges.
From
Aluminum Charges aluminium has a charge of +3. Aluminium has 13 electrons and 13 protons. the charge of an aluminum ion is typically 3+. These electrons are distributed across the shells in the. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as.. Aluminum Charges.
From studylibrarygodward.z13.web.core.windows.net
How To Write Charge Of An Ion Aluminum Charges the charge of an aluminum ion is typically 3+. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. This is because the element’s atomic number is 13, reflecting the fact. aluminium has a charge of +3. All group 17 elements (halogens) gain one. using a simple, general trend for the. Aluminum Charges.
From sendcutsend.com
How to Identify the Difference in Aluminum Alloys Aluminum Charges All group 17 elements (halogens) gain one. These electrons are distributed across the shells in the. the charge of an aluminum ion is typically 3+. Aluminium has 13 electrons and 13 protons. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. This is because the element’s atomic number is 13, reflecting the. Aluminum Charges.
From
Aluminum Charges during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. These electrons are distributed across the shells in the. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. This is because the element’s atomic number is 13, reflecting the fact. Aluminium has 13 electrons and 13. Aluminum Charges.
From
Aluminum Charges 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. These electrons are distributed across the shells in the. Aluminium has 13 electrons and 13 protons. . Aluminum Charges.
From
Aluminum Charges This is because the element’s atomic number is 13, reflecting the fact. These electrons are distributed across the shells in the. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. All group 17 elements (halogens) gain. Aluminum Charges.
From www.museoinclusivo.com
Aluminum Charging Exploring the Benefits, Types and Uses Aluminum Aluminum Charges These electrons are distributed across the shells in the. This is because the element’s atomic number is 13, reflecting the fact. the charge of an aluminum ion is typically 3+. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. All group. Aluminum Charges.
From
Aluminum Charges Aluminium has 13 electrons and 13 protons. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. the charge of an aluminum ion is typically 3+.. Aluminum Charges.
From
Aluminum Charges Aluminium has 13 electrons and 13 protons. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. These electrons are distributed across the shells in the. using a simple, general trend for the ionic charge for elements on the. 93 rows for example, group i elements on the periodic table, known. Aluminum Charges.
From
Aluminum Charges aluminium has a charge of +3. These electrons are distributed across the shells in the. All group 17 elements (halogens) gain one. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. using a simple, general trend for the ionic charge. Aluminum Charges.
From www.museoinclusivo.com
Aluminum Charging Exploring the Benefits, Types and Uses Aluminum Aluminum Charges Aluminium has 13 electrons and 13 protons. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. 93 rows for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii elements, referred to as. using a simple, general trend for the ionic charge. Aluminum Charges.
From
Aluminum Charges These electrons are distributed across the shells in the. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. Aluminium has 13 electrons and 13 protons. using a simple, general trend for the ionic charge for elements on the. All group 17 elements (halogens) gain one. 93 rows for example, group i. Aluminum Charges.
From
Aluminum Charges the charge of an aluminum ion is typically 3+. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. during the chemical reaction, aluminum loses these 3 electrons and achieves the nearest noble gas. All group 17 elements (halogens) gain one. This is because the element’s atomic number is 13, reflecting. Aluminum Charges.
From getecna.com
Designing Aluminum Extrusions Getec Industrial Aluminum Charges Aluminium has 13 electrons and 13 protons. group 13 metals like aluminum lose three electrons to form an ion with a 3+ charge. These electrons are distributed across the shells in the. This is because the element’s atomic number is 13, reflecting the fact. 93 rows for example, group i elements on the periodic table, known as alkali. Aluminum Charges.
From
Aluminum Charges Aluminium has 13 electrons and 13 protons. aluminium has a charge of +3. the charge of an aluminum ion is typically 3+. This is because the element’s atomic number is 13, reflecting the fact. These electrons are distributed across the shells in the. using a simple, general trend for the ionic charge for elements on the. . Aluminum Charges.