A Box With A Volume Of 22.4 L Contains . The partial pressure is the pressure that a gas and a. 5 people found it helpful. A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. Which of the following statements is true? The question gives us the ability to determine if they're true or false. A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. Which of the following statements is true? A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees celsius. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. The partial pressure of n2 is 101 kpa.
from www.chegg.com
A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. The partial pressure of n2 is 101 kpa. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. The partial pressure is the pressure that a gas and a. A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees celsius. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. Which of the following statements is true? A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. 5 people found it helpful.
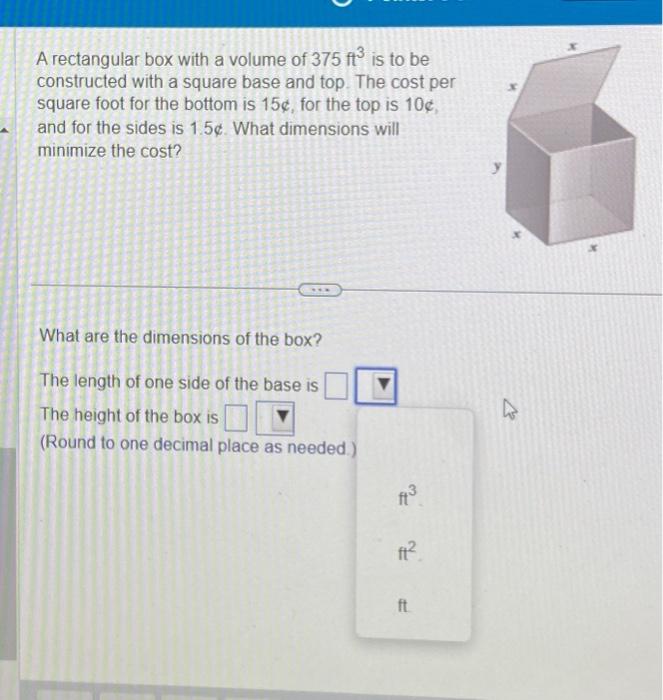
Solved A rectangular box with a volume of 375ft3 is to be
A Box With A Volume Of 22.4 L Contains A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. The partial pressure is the pressure that a gas and a. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. Which of the following statements is true? A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. The question gives us the ability to determine if they're true or false. 5 people found it helpful. The partial pressure of n2 is 101 kpa. A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees celsius. Which of the following statements is true?
From www.chegg.com
Solved Q2). A box with a volume of 1000 cm3 is to be made in A Box With A Volume Of 22.4 L Contains 5 people found it helpful. A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. The partial pressure of n2 is 101 kpa. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. Which of the following statements is true? A box. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A box with an open top is to be constructed from a A Box With A Volume Of 22.4 L Contains The partial pressure is the pressure that a gas and a. A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. The question gives us the ability to determine if they're true or false. Which of the following statements is true? A box with a volume of. A Box With A Volume Of 22.4 L Contains.
From www.numerade.com
SOLVEDMinimizing costs. A rectangular box with a volume of 320 ft^3 is A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. The question gives us the ability to determine if they're true or false. Which of the following. A Box With A Volume Of 22.4 L Contains.
From www.coursehero.com
[Solved] An open rectangular box with a square base of sidelength "x A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. Which of the following statements is true? The partial pressure of n2 is 101 kpa. A box. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 1280 ft^3 is to be A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. Which of the following statements is true? The question gives us the ability to determine if they're true or false. A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved 4. a)What is the volume of a box where the length of A Box With A Volume Of 22.4 L Contains A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees celsius. A box. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 76 ft3 is to be A Box With A Volume Of 22.4 L Contains Which of the following statements is true? The partial pressure is the pressure that a gas and a. The partial pressure of n2 is 101 kpa. A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of. A Box With A Volume Of 22.4 L Contains.
From www.youtube.com
Volume of a Box 1 YouTube A Box With A Volume Of 22.4 L Contains The partial pressure is the pressure that a gas and a. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees celsius. Which of the following statements is true? The. A Box With A Volume Of 22.4 L Contains.
From www.youtube.com
Finding the maximum volume of a box YouTube A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. The partial pressure of n2 is 101 kpa. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with a volume of 22.4 l contains 2.0 mol. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 375ft3 is to be A Box With A Volume Of 22.4 L Contains The partial pressure is the pressure that a gas and a. The partial pressure of n2 is 101 kpa. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. 5 people found it helpful. A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and. A Box With A Volume Of 22.4 L Contains.
From www.youtube.com
A box with an open top is to be constructed from a rectangular piece of A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. 5 people found it helpful. Which of the following statements is true? The question gives us. A Box With A Volume Of 22.4 L Contains.
From dennis-relmay.blogspot.com
A CLOSED RECTANGULAR BOX WITH A VOLUME OF 16 DennisRelMay A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. The partial pressure is the pressure that a gas and a. A box with a volume of 22.4 l. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A box with a square base and open top must have a A Box With A Volume Of 22.4 L Contains 5 people found it helpful. The partial pressure is the pressure that a gas and a. Which of the following statements is true? The question gives us the ability to determine if they're true or false. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with a. A Box With A Volume Of 22.4 L Contains.
From www.youtube.com
A rectangular storage container with an open top is to have a volume of A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. 5 people found it helpful. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. A box with a volume of 22.4 l contains 1.0 mol. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A box with a square base and an open top must have a A Box With A Volume Of 22.4 L Contains 5 people found it helpful. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. The partial pressure is the pressure that a gas and a. A box with a volume. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 735ft3 is to be A Box With A Volume Of 22.4 L Contains 5 people found it helpful. The partial pressure is the pressure that a gas and a. The partial pressure of n2 is 101 kpa. A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees celsius. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 76ft3 is to be A Box With A Volume Of 22.4 L Contains A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees celsius. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved Consider a rectangular box with a volume of 32 ft3 A Box With A Volume Of 22.4 L Contains A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees celsius. A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 960 ft3 is to be A Box With A Volume Of 22.4 L Contains The question gives us the ability to determine if they're true or false. The partial pressure of n2 is 101 kpa. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved 3. What is the volume of this box? 5.0 cm 4.0 cm 6.0 A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. Which of the following statements is true? The partial pressure of n2 is 101 kpa. A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. A box with a volume of. A Box With A Volume Of 22.4 L Contains.
From www.teachoo.com
Example 4 Find the volume of each rectangular box with given length A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. The partial pressure of n2 is 101 kpa. 5 people found it helpful. Which of the following statements is true? A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 425ft3 is to be A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. 5 people found it helpful. The question gives us the ability to determine if they're true or false. The partial pressure is. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 240ft3 is to be A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. Which of the following statements is true? A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. The partial pressure is the pressure that a gas. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 720 ft is to be A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. Which of the following statements is true? The question gives us the ability to determine if. A Box With A Volume Of 22.4 L Contains.
From earnca.com
Volume of a Square Box Formula, Definition, Examples A Box With A Volume Of 22.4 L Contains A box with avolume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol ofhydrogen at 0°c. The partial pressure of n2 is 101 kpa. The question gives us the ability to determine if they're true or false. The partial pressure is the pressure that a gas and a. A box with a volume of 22.4 l contains 1.0. A Box With A Volume Of 22.4 L Contains.
From alternativedirection12.bitbucket.io
How To Find Out The Volume Of A Box Alternativedirection12 A Box With A Volume Of 22.4 L Contains 5 people found it helpful. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. The question gives us the ability to determine if they're true or false. A. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved 2 a A box with a volume of 1000 cm3 is to be made in A Box With A Volume Of 22.4 L Contains Which of the following statements is true? The question gives us the ability to determine if they're true or false. A box with a vlume of 22.4 l contains 1.0 mol nitrogen and 2.0 mol hydrogen at 0 degrees celsius. The partial pressure is the pressure that a gas and a. The partial pressure of n2 is 101 kpa. Which. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 80ft3 is to be A Box With A Volume Of 22.4 L Contains The question gives us the ability to determine if they're true or false. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with. A Box With A Volume Of 22.4 L Contains.
From brainly.com
If a total of 1700 square centimeters of material is to be used to make A Box With A Volume Of 22.4 L Contains Which of the following statements is true? Which of the following statements is true? A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with. A Box With A Volume Of 22.4 L Contains.
From www.numerade.com
SOLVED Solve the problem A company wishes to manufacture box with A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. The question gives us the ability to determine if they're true or false. The partial pressure is the pressure that a gas and a. A box with a volume of 22.4l contains 1.0 mol of nitrogen and. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 320 ft is to be A Box With A Volume Of 22.4 L Contains 5 people found it helpful. The question gives us the ability to determine if they're true or false. The partial pressure of n2 is 101 kpa. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. Which of the following statements is true? The partial pressure is the pressure. A Box With A Volume Of 22.4 L Contains.
From www.coursehero.com
[Solved] Find the dimensions of the open rectangular box of maximum A Box With A Volume Of 22.4 L Contains 5 people found it helpful. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. Which of the following statements is true? The partial pressure of n2 is 101 kpa. The partial pressure is the pressure that a gas and a. A box with a volume of 22.4l contains. A Box With A Volume Of 22.4 L Contains.
From www.numerade.com
SOLVEDpoints) A box with an open top is to be constructed from a A Box With A Volume Of 22.4 L Contains The partial pressure is the pressure that a gas and a. A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0° c. The question gives us the. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A box with a volume of 21, contains 6 molecules. Now A Box With A Volume Of 22.4 L Contains Which of the following statements is true? The question gives us the ability to determine if they're true or false. A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. The partial pressure of n2 is 101 kpa. A box with a volume of 22.4 l contains. A Box With A Volume Of 22.4 L Contains.
From www.chegg.com
Solved A rectangular box with a volume of 23 ft has a square A Box With A Volume Of 22.4 L Contains A box with a volume of 22.4l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0 c. A box with a volume of 22.4 l contains 2.0 mol of nitrogen gas and 1.0 mol of hydrogen gas at 0 c. A box with a volume of 22.4 l contains 1.0 mol of nitrogen and 2.0 mol of. A Box With A Volume Of 22.4 L Contains.