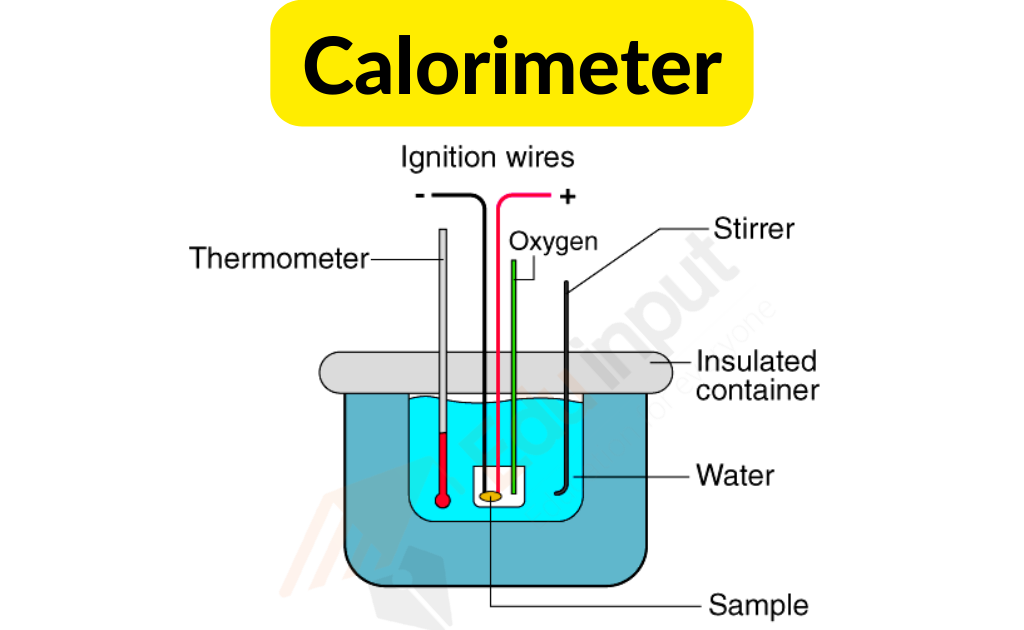
Calorimeter Instrumentation . Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimeters have been designed in great variety. Isothermal, isoperibol, and adiabatic are three. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Fundamentals, instrumentation and applications, first edition. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. The underlying principle is that the heat emitted by the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials.
from eduinput.com
Apply the first law of thermodynamics to calorimetry. Calorimeters have been designed in great variety. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. The underlying principle is that the heat emitted by the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Fundamentals, instrumentation and applications, first edition. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Isothermal, isoperibol, and adiabatic are three.
CalorimeterDefinition, History, Construction, Types, And Uses
Calorimeter Instrumentation The underlying principle is that the heat emitted by the. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimeters have been designed in great variety. The underlying principle is that the heat emitted by the. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Isothermal, isoperibol, and adiabatic are three. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Fundamentals, instrumentation and applications, first edition. Apply the first law of thermodynamics to calorimetry.
From www.indiamart.com
Junkers Gas Calorimeter, For Lab, Automation Grade Automatic, ID Calorimeter Instrumentation Fundamentals, instrumentation and applications, first edition. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The underlying principle is that the heat emitted by the. Apply the first law of thermodynamics. Calorimeter Instrumentation.
From www.slideshare.net
Differential Scanning Calorimeter Instrumentation.(DSC) Calorimeter Instrumentation A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in. Calorimeter Instrumentation.
From edulab.com
Calorimeter Set, Aluminium Edulab Edulab Calorimeter Instrumentation A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calorimeters have been designed in great variety. Isothermal, isoperibol, and adiabatic are three. Fundamentals, instrumentation and applications, first edition. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Apply the first law of thermodynamics to calorimetry.. Calorimeter Instrumentation.
From www.slideshare.net
Differential Scanning Calorimeter Instrumentation.(DSC) PPT Calorimeter Instrumentation Apply the first law of thermodynamics to calorimetry. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Isothermal, isoperibol, and adiabatic are three. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an. Calorimeter Instrumentation.
From chemistry.humboldt.edu
Instrumentation Department of Chemistry Calorimeter Instrumentation A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Fundamentals, instrumentation and applications, first edition. The underlying principle is that the heat emitted by the. Isothermal, isoperibol, and adiabatic are three. Calorimeters have been designed in great variety. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure. Calorimeter Instrumentation.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Instrumentation Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Fundamentals, instrumentation and applications, first edition. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Apply the first law of thermodynamics to calorimetry. Isothermal, isoperibol, and adiabatic. Calorimeter Instrumentation.
From lumigen.wayne.edu
Optical spectroscopy and calorimeter instrumentation Lumigen Calorimeter Instrumentation Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Fundamentals, instrumentation and applications, first edition. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed. Calorimeter Instrumentation.
From www.motistech.com
Bomb Calorimeter ISO 1716 Calorimeter Instrumentation Isothermal, isoperibol, and adiabatic are three. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Fundamentals, instrumentation and applications, first edition. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. A calorimeter is a device that. Calorimeter Instrumentation.
From www.fishersci.com
Parr 1341 Plain Jacket CalorimeterSpecialty Lab Equipment, Instruments Calorimeter Instrumentation Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Fundamentals, instrumentation and applications, first edition. A calorimeter is a device that measures the heat flow produced by a chemical. Calorimeter Instrumentation.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Instrumentation A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Fundamentals, instrumentation and applications, first edition. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Apply the first law of thermodynamics to calorimetry. The underlying principle is that the heat emitted. Calorimeter Instrumentation.
From www.shutterstock.com
5 Bomb Calorimeter Instrumentation RoyaltyFree Images, Stock Photos Calorimeter Instrumentation The underlying principle is that the heat emitted by the. Isothermal, isoperibol, and adiabatic are three. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Compare heat. Calorimeter Instrumentation.
From www.walmart.com
Electric Calorimeter Calorimeter Instrumentation A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Fundamentals, instrumentation and applications,. Calorimeter Instrumentation.
From www.terraanaliz.com
Atlas HD Reaction Calorimeter Batch Chemistry Calorimeter Instrumentation The underlying principle is that the heat emitted by the. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device that measures the heat flow produced by a chemical. Calorimeter Instrumentation.
From fbri.vtc.vt.edu
Whole Room Calorimeters Fralin Biomedical Research Institute at VTC Calorimeter Instrumentation Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a.. Calorimeter Instrumentation.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Instrumentation Apply the first law of thermodynamics to calorimetry. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Isothermal, isoperibol, and. Calorimeter Instrumentation.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Calorimeter Instrumentation A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Fundamentals, instrumentation and applications, first edition. Apply the first law of thermodynamics to calorimetry. Isothermal, isoperibol, and adiabatic are three. Calorimeters have been designed in great variety. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change.. Calorimeter Instrumentation.
From mcf.tamu.edu
Differential Scanning Calorimeter (DSC) Materials Characterization Calorimeter Instrumentation Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Apply the first law of thermodynamics to calorimetry. Isothermal, isoperibol, and adiabatic are three. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Fundamentals, instrumentation and applications, first edition. Calorimeters have. Calorimeter Instrumentation.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Instrumentation Fundamentals, instrumentation and applications, first edition. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Isothermal, isoperibol, and adiabatic are three. Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal. Calorimeter Instrumentation.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimeter Instrumentation Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Compare heat flow from hot to cold objects in. Calorimeter Instrumentation.
From www.3bscientific.co.uk
Copper Calorimeter 1002659 U10366 Calorimeters 3B Scientific Calorimeter Instrumentation Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Isothermal, isoperibol, and adiabatic are three. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Fundamentals, instrumentation and. Calorimeter Instrumentation.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Instrumentation Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Calorimeters have been designed in great variety. A calorimeter is a device that measures. Calorimeter Instrumentation.
From analyzing-testing.netzsch.com
Accelerating Rate Calorimeter 244 (ARC®) NETZSCH Analyzing & Testing Calorimeter Instrumentation The underlying principle is that the heat emitted by the. Fundamentals, instrumentation and applications, first edition. Apply the first law of thermodynamics to calorimetry. Isothermal, isoperibol, and adiabatic are three. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Calculate heat, temperature change, and specific heat after thermal equilibrium. Calorimeter Instrumentation.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Calorimeter Instrumentation Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device that measures the heat flow. Calorimeter Instrumentation.
From www.3bscientific.com
Calorimeter with Heating Coil, 150 ml 1000822 U8441020 Calorimeter Instrumentation A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The underlying principle is that the heat emitted by the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction. Calorimeter Instrumentation.
From www.directindustry.com
Isothermal calorimeter Atlas HD Syrris reaction Calorimeter Instrumentation A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Fundamentals, instrumentation and applications, first edition. Calorimeters have been designed in great variety. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Compare heat flow from hot to cold objects in an ideal calorimeter. Calorimeter Instrumentation.
From www.ebay.com
TA INSTRUMENTS DSC Q100 DIFFERENTIAL SCANNING CALORIMETER MDSC(R) MFC w Calorimeter Instrumentation Calorimeters have been designed in great variety. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. The underlying principle is that the heat emitted by the. Compare heat flow from hot to. Calorimeter Instrumentation.
From analyzing-testing.netzsch.com
Accelerating Rate Calorimeter 254 (ARC) NETZSCH Analyzing & Testing Calorimeter Instrumentation Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. Isothermal, isoperibol, and adiabatic are three. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter is an instrument used to determine the heat exchange rate. Calorimeter Instrumentation.
From www.slideshare.net
Differential Scanning Calorimeter Instrumentation.(DSC) PPT Calorimeter Instrumentation Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The underlying principle is that the heat emitted. Calorimeter Instrumentation.
From www.researchgate.net
Types of differential scanning calorimeters (a) heatflux calorimeter Calorimeter Instrumentation Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Apply the first law of thermodynamics to calorimetry. Isothermal, isoperibol, and adiabatic are three. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters have been designed. Calorimeter Instrumentation.
From saylordotorg.github.io
Calorimetry Calorimeter Instrumentation Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter is an instrument used to determine the heat exchange rate and heat capacity. Calorimeters have been designed in. Calorimeter Instrumentation.
From www.youtube.com
Differential Scanning Calorimetry DSC Principle Instrumentation Calorimeter Instrumentation Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Isothermal, isoperibol, and adiabatic are three. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. Calorimeter Instrumentation.
From labsuppliesusa.com
Calorimeter Electric KLM Bio Scientific Calorimeter Instrumentation Fundamentals, instrumentation and applications, first edition. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Apply the first law of thermodynamics to calorimetry. Isothermal, isoperibol, and adiabatic are three.. Calorimeter Instrumentation.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimeter Instrumentation Apply the first law of thermodynamics to calorimetry. The underlying principle is that the heat emitted by the. Calorimeters have been designed in great variety. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Fundamentals, instrumentation and applications, first edition. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis. Calorimeter Instrumentation.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Instrumentation A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. Apply the first law of thermodynamics to calorimetry. Isothermal, isoperibol, and adiabatic are three. Calculate heat, temperature change, and specific heat after. Calorimeter Instrumentation.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Instrumentation Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Differential scanning calorimeters (dsc) are scientific instruments used in thermal analysis to measure the energy absorbed or released by a. The underlying principle is that the heat emitted by the. Calorimeters have been designed in great variety. Fundamentals,. Calorimeter Instrumentation.