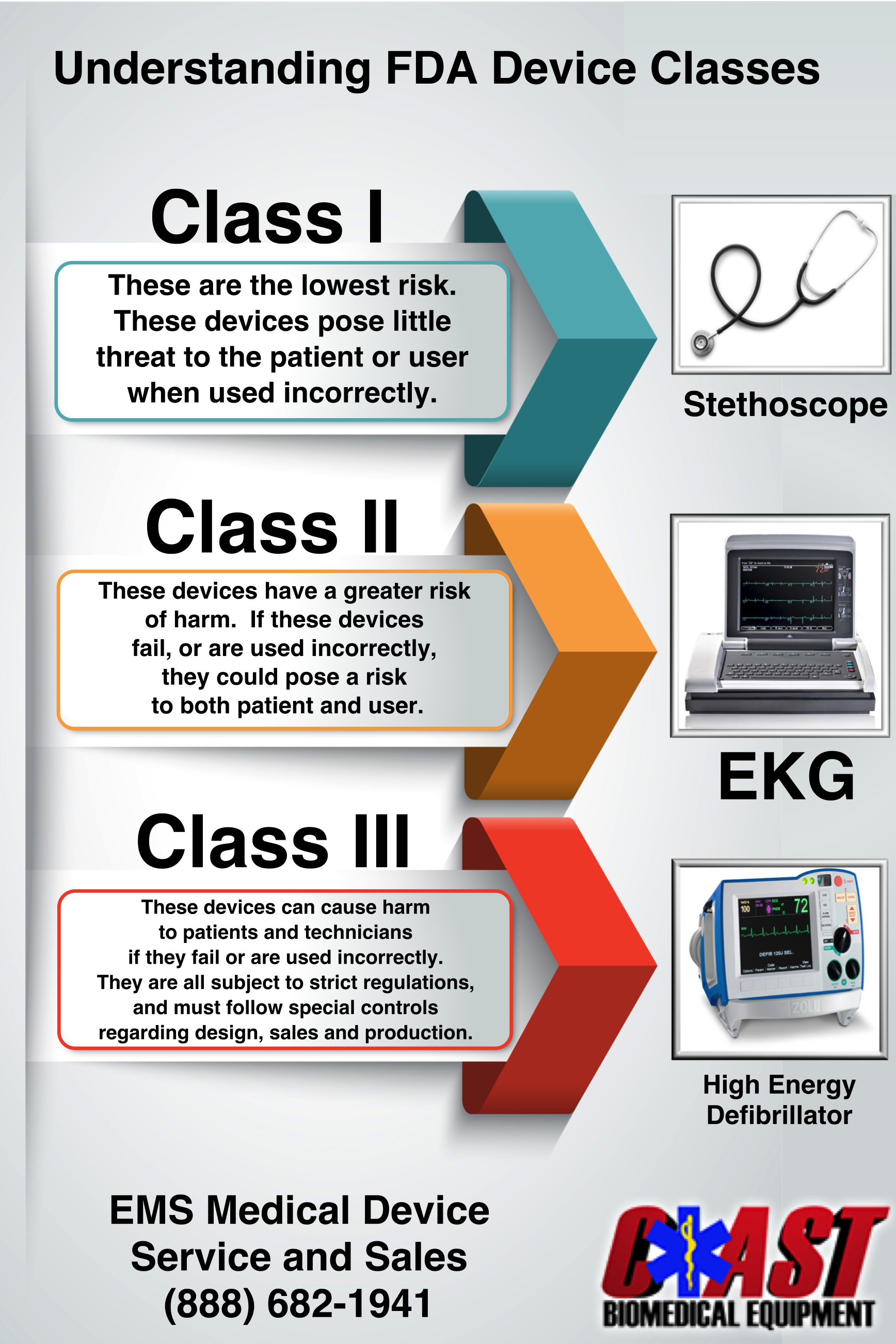
Medical Devices Definition According To Who . (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by.
from coastbiomed.com
(1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,.
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment
Medical Devices Definition According To Who As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Medical Devices Definition According To Who “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. A medical device is. Medical Devices Definition According To Who.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Devices Definition According To Who An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by.. Medical Devices Definition According To Who.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Devices Definition According To Who “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. To this purpose, three. Medical Devices Definition According To Who.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Definition According To Who (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. To this purpose, three publications were. Medical Devices Definition According To Who.
From medicaldevicehq.com
What is a medical device? Medical Device HQ Medical Devices Definition According To Who (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. To this purpose, three publications. Medical Devices Definition According To Who.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Devices Definition According To Who To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. As stated in document eb148/13, medical devices, including. Medical Devices Definition According To Who.
From mavink.com
Examples Of Medical Devices Medical Devices Definition According To Who An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. A medical device is an article, instrument, apparatus. Medical Devices Definition According To Who.
From premier-research.com
Medical Device Trials What You Need to Know About U.S. Regulations Medical Devices Definition According To Who To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. (1) ‘medical device’ means any instrument,. Medical Devices Definition According To Who.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Medical Devices Definition According To Who As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. “medical devices are essential for safe. Medical Devices Definition According To Who.
From laegemiddelstyrelsen.dk
Medical devices Medical Devices Definition According To Who “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device. Medical Devices Definition According To Who.
From www.slideserve.com
PPT The FDA Landscape AdvaMed September 2008 PowerPoint Presentation Medical Devices Definition According To Who As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. An article, instrument, apparatus or machine that. Medical Devices Definition According To Who.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Devices Definition According To Who (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. To this purpose, three. Medical Devices Definition According To Who.
From www.slideserve.com
PPT FDA Medical Device Rules PowerPoint Presentation, free download Medical Devices Definition According To Who An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. As stated in document eb148/13, medical devices, including. Medical Devices Definition According To Who.
From medicaldevicehq.com
What is a medical device according to the MDR Medical Device HQ 1 Medical Devices Definition According To Who An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. A medical device is an. Medical Devices Definition According To Who.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Devices Definition According To Who (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. To this purpose, three publications were initially. Medical Devices Definition According To Who.
From www.qualitymeddev.com
Medical Device Definition according to EU MDR 2017/745 Medical Devices Definition According To Who “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. A medical device is an article,. Medical Devices Definition According To Who.
From mungfali.com
Classification Of Medical Devices Medical Devices Definition According To Who To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. An article, instrument, apparatus or machine that is used in. Medical Devices Definition According To Who.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Definition According To Who To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. An article, instrument, apparatus. Medical Devices Definition According To Who.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Medical Devices Definition According To Who An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,.. Medical Devices Definition According To Who.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Devices Definition According To Who As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. A medical device is an article, instrument,. Medical Devices Definition According To Who.
From www.researchgate.net
3 Examples of medical devices according to the European and USA Medical Devices Definition According To Who To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. “medical devices are. Medical Devices Definition According To Who.
From www.youtube.com
What are medical devices? YouTube Medical Devices Definition According To Who “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. To this purpose, three. Medical Devices Definition According To Who.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Devices Definition According To Who (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. “medical devices are essential for safe and. Medical Devices Definition According To Who.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Medical Devices Definition According To Who As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. “medical devices are essential for safe. Medical Devices Definition According To Who.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) Medical Devices Definition According To Who As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by.. Medical Devices Definition According To Who.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Devices Definition According To Who “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. As stated in document eb148/13, medical devices, including. Medical Devices Definition According To Who.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation ID254282 Medical Devices Definition According To Who (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. A medical device is an. Medical Devices Definition According To Who.
From woodleybioreg.com
Medical devices what are they, and how do we know that they're safe Medical Devices Definition According To Who An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. As stated in document. Medical Devices Definition According To Who.
From www.arenasolutions.com
Medical Device Definition Arena Medical Devices Definition According To Who “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. (1) ‘medical device’. Medical Devices Definition According To Who.
From mavink.com
Examples Of Medical Devices Medical Devices Definition According To Who An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. As stated in document. Medical Devices Definition According To Who.
From de.slideshare.net
Medical device definition Medical Devices Definition According To Who (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. To this purpose, three publications were initially conceptualized and developed to define the medical devices required for essential. An article, instrument, apparatus or machine. Medical Devices Definition According To Who.
From mexicobusiness.news
Classification of Medical Devices According to Risk Medical Devices Definition According To Who An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. (1) ‘medical device’. Medical Devices Definition According To Who.
From www.slideserve.com
PPT Medical Devices PowerPoint Presentation, free download ID991833 Medical Devices Definition According To Who An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,.. Medical Devices Definition According To Who.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Devices Definition According To Who As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by.. Medical Devices Definition According To Who.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Devices Definition According To Who “medical devices are essential for safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease. As stated in document eb148/13, medical devices, including in vitro diagnostic medical devices, are health products that are required for protection,. A medical device is an article, instrument, apparatus or machine (including mobile medical applications and software) that is intended by. To this. Medical Devices Definition According To Who.