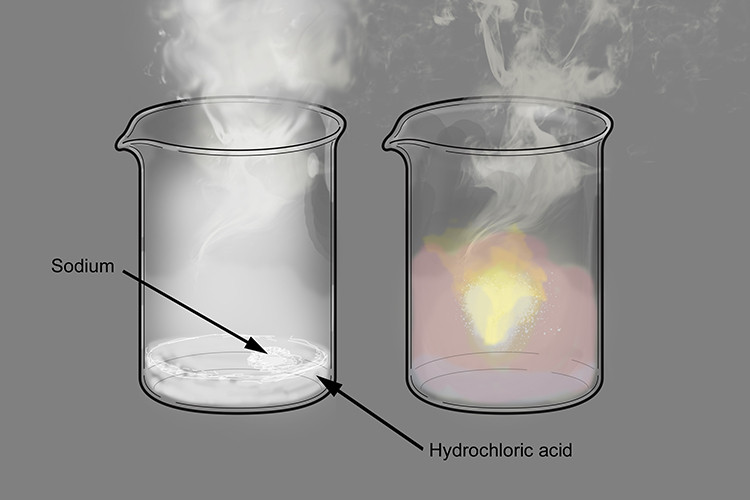
Copper Chloride Reacts With Hydrochloric Acid . copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. It is above copper in a metal reactivity series, so copper cannot. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. you can think of this happening in two stages. copper is a very unreactive metal, and it does not react with hydrochloric acid. First, you get copper(i) chloride formed: On the other hand, sodium is too reactive. for example, copper does not react with dilute acids, so this metal cannot be used. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of.
from www.vrogue.co
First, you get copper(i) chloride formed: copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. It is above copper in a metal reactivity series, so copper cannot. On the other hand, sodium is too reactive. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. you can think of this happening in two stages. copper is a very unreactive metal, and it does not react with hydrochloric acid. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. for example, copper does not react with dilute acids, so this metal cannot be used.
Sodium Metal Reacts With Hydrochloric Acid vrogue.co
Copper Chloride Reacts With Hydrochloric Acid On the other hand, sodium is too reactive. On the other hand, sodium is too reactive. you can think of this happening in two stages. as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of. copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. copper is a very unreactive metal, and it does not react with hydrochloric acid. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. First, you get copper(i) chloride formed: It is above copper in a metal reactivity series, so copper cannot. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. for example, copper does not react with dilute acids, so this metal cannot be used.
From mmerevise.co.uk
Reactions of Acids Worksheets and Revision MME Copper Chloride Reacts With Hydrochloric Acid so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. copper oxide (cuo) (c u o). Copper Chloride Reacts With Hydrochloric Acid.
From www.slideshare.net
Metals Reactivity Series Copper Chloride Reacts With Hydrochloric Acid however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. you can think of this happening in two stages. It is above copper in a metal reactivity series, so copper cannot. for example, copper does not react with dilute acids, so this metal cannot be used. so, copper reacts with the. Copper Chloride Reacts With Hydrochloric Acid.
From www.chegg.com
Solved F. Other Reactions 14. Copper(II)chloride and water Copper Chloride Reacts With Hydrochloric Acid you can think of this happening in two stages. for example, copper does not react with dilute acids, so this metal cannot be used. On the other hand, sodium is too reactive. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. however, the situation is slightly. Copper Chloride Reacts With Hydrochloric Acid.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper Chloride Reacts With Hydrochloric Acid so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. On the other hand, sodium is too reactive. First, you get copper(i) chloride formed: It is above copper in a metal reactivity series, so. Copper Chloride Reacts With Hydrochloric Acid.
From www.researchgate.net
(PDF) Effect of a pretreatment with hydrochloric acid on the copper Copper Chloride Reacts With Hydrochloric Acid copper is a very unreactive metal, and it does not react with hydrochloric acid. as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of. First, you get copper(i) chloride formed: On the other hand, sodium is too reactive. copper oxide (cuo) (c u o) reacts with hydrochloric. Copper Chloride Reacts With Hydrochloric Acid.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Copper Chloride Reacts With Hydrochloric Acid It is above copper in a metal reactivity series, so copper cannot. copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. you can think of this happening in two stages. copper is a very unreactive metal, and it does not react with hydrochloric acid. On the other hand, sodium is. Copper Chloride Reacts With Hydrochloric Acid.
From spmscience.blog.onlinetuition.com.my
Electrolysis of Copper (II) Chloride Solution SPM Science Copper Chloride Reacts With Hydrochloric Acid copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. for example, copper does not react with dilute acids, so this metal cannot be used. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. as. Copper Chloride Reacts With Hydrochloric Acid.
From express.adobe.com
Zinc and Copper Chloride Reaction Copper Chloride Reacts With Hydrochloric Acid copper is a very unreactive metal, and it does not react with hydrochloric acid. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of. copper(i) chloride.is a white solid sparingly soluble. Copper Chloride Reacts With Hydrochloric Acid.
From exyizoopg.blob.core.windows.net
Chlorine Dioxide Synthesis at Bradley Tesch blog Copper Chloride Reacts With Hydrochloric Acid for example, copper does not react with dilute acids, so this metal cannot be used. First, you get copper(i) chloride formed: copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking. Copper Chloride Reacts With Hydrochloric Acid.
From hxetahfoe.blob.core.windows.net
Copper Chloride Reaction With Iron at Pauline Estill blog Copper Chloride Reacts With Hydrochloric Acid however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. copper is a very unreactive metal, and it does not react with hydrochloric acid. for example, copper does not react with dilute acids, so this metal cannot be used. On the other hand, sodium is too reactive. It is above copper in. Copper Chloride Reacts With Hydrochloric Acid.
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Copper Chloride Reacts With Hydrochloric Acid as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of. copper is a very unreactive metal, and it does not react with hydrochloric acid. It is above copper in a metal reactivity series, so copper cannot. however, the situation is slightly complicated because of the low solubility. Copper Chloride Reacts With Hydrochloric Acid.
From www.youtube.com
Copper & Hydrochloric Acid Ligand Substitution YouTube Copper Chloride Reacts With Hydrochloric Acid for example, copper does not react with dilute acids, so this metal cannot be used. It is above copper in a metal reactivity series, so copper cannot. you can think of this happening in two stages. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. however,. Copper Chloride Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper Chloride Reacts With Hydrochloric Acid so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. you can think of this happening in two stages. First, you get copper(i) chloride. Copper Chloride Reacts With Hydrochloric Acid.
From www.youtube.com
Ag + HCl (Silver + Hydrochloric acid) Equation YouTube Copper Chloride Reacts With Hydrochloric Acid It is above copper in a metal reactivity series, so copper cannot. as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of. First, you get copper(i) chloride formed: On the other hand, sodium is too reactive. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl). Copper Chloride Reacts With Hydrochloric Acid.
From spark.adobe.com
Zinc and Copper Chloride Reaction Copper Chloride Reacts With Hydrochloric Acid so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. First, you get copper(i) chloride formed: you can think of this happening in two stages. copper is a very unreactive metal, and it does not react with hydrochloric acid. copper oxide (cuo) (c u o) reacts with. Copper Chloride Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED How many grams of copper (I) chloride are formed if 5.000 g of Copper Chloride Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. for example, copper does not react with dilute acids, so this metal cannot be. Copper Chloride Reacts With Hydrochloric Acid.
From www.slideshare.net
Chemical Reactions Copper Chloride Reacts With Hydrochloric Acid as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$. Copper Chloride Reacts With Hydrochloric Acid.
From www.youtube.com
Hydrochloric Acid Reaction HCL and mixed metal copper oxides YouTube Copper Chloride Reacts With Hydrochloric Acid you can think of this happening in two stages. copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of. copper is a very unreactive metal, and it does not react. Copper Chloride Reacts With Hydrochloric Acid.
From brainly.com
The diagram below shows a stage in the preparation of copper chloride Copper Chloride Reacts With Hydrochloric Acid On the other hand, sodium is too reactive. copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. First, you get copper(i) chloride formed: for example, copper does not react with dilute. Copper Chloride Reacts With Hydrochloric Acid.
From www.youtube.com
Reaction of Copper Chloride with Aluminum Best Demonstration of Copper Chloride Reacts With Hydrochloric Acid so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. for example, copper does not react with dilute acids, so this metal cannot be used. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. copper oxide (cuo) (c u o) reacts. Copper Chloride Reacts With Hydrochloric Acid.
From kolblabs.com
Color with cupric chloride reaction in Structure Of Atom Class 10 Copper Chloride Reacts With Hydrochloric Acid so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. for example, copper does not react with dilute acids, so this metal cannot be used. as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of. however, the. Copper Chloride Reacts With Hydrochloric Acid.
From tatum-kharrington.blogspot.com
Which Best Describes an Aqueous Solution of Copper Chloride Copper Chloride Reacts With Hydrochloric Acid however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. for example, copper does not react with dilute acids, so this metal cannot be used. copper is a very unreactive metal, and it does not react with hydrochloric acid. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl). Copper Chloride Reacts With Hydrochloric Acid.
From exobqbaxs.blob.core.windows.net
Copper Chloride Reaction With Water at Florence McIntosh blog Copper Chloride Reacts With Hydrochloric Acid for example, copper does not react with dilute acids, so this metal cannot be used. copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. you can think of this happening in two stages. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to. Copper Chloride Reacts With Hydrochloric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Copper Chloride Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. as ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's. Copper Chloride Reacts With Hydrochloric Acid.
From studylib.net
Laboratory 5 Stoichiometry of Copper (II) Chloride and Aluminum Copper Chloride Reacts With Hydrochloric Acid you can think of this happening in two stages. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. copper is a very unreactive metal, and it does not react with hydrochloric acid. for example, copper does not react with dilute acids, so this metal cannot be. Copper Chloride Reacts With Hydrochloric Acid.
From exobqbaxs.blob.core.windows.net
Copper Chloride Reaction With Water at Florence McIntosh blog Copper Chloride Reacts With Hydrochloric Acid First, you get copper(i) chloride formed: however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. On the other hand, sodium is too reactive. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. so, copper reacts with. Copper Chloride Reacts With Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with Copper Chloride Reacts With Hydrochloric Acid copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. On the other hand, sodium is too reactive. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. copper is a very unreactive metal, and it does not react with hydrochloric acid.. Copper Chloride Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVEDZinc and magnesium metal each reacts with hydrochloric acid to Copper Chloride Reacts With Hydrochloric Acid so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. you can think of this happening in two stages. On the other hand, sodium is too reactive. First, you get copper(i) chloride. Copper Chloride Reacts With Hydrochloric Acid.
From www.vrogue.co
Sodium Metal Reacts With Hydrochloric Acid vrogue.co Copper Chloride Reacts With Hydrochloric Acid First, you get copper(i) chloride formed: copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. On the other hand, sodium is too reactive. copper is a very unreactive metal, and it does not react with hydrochloric acid. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h. Copper Chloride Reacts With Hydrochloric Acid.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Copper Chloride Reacts With Hydrochloric Acid however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. so, copper reacts with the hydrochloric acid, replacing the hydrogen and taking it's place in the acid, forming the. you can think of this happening in two stages. copper is a very unreactive metal, and it does not react with hydrochloric. Copper Chloride Reacts With Hydrochloric Acid.
From copperlabgroup11.blogspot.com
Copper Lab Copper Chloride Reacts With Hydrochloric Acid copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. It is above copper in a metal reactivity series, so copper cannot. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. for example, copper does not react. Copper Chloride Reacts With Hydrochloric Acid.
From www.alamy.com
Copper chloride reaction hires stock photography and images Alamy Copper Chloride Reacts With Hydrochloric Acid however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. It is above copper in a metal reactivity series, so copper cannot. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. you can think of this happening. Copper Chloride Reacts With Hydrochloric Acid.
From exobqbaxs.blob.core.windows.net
Copper Chloride Reaction With Water at Florence McIntosh blog Copper Chloride Reacts With Hydrochloric Acid copper is a very unreactive metal, and it does not react with hydrochloric acid. however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c u c. copper(i) chloride.is a. Copper Chloride Reacts With Hydrochloric Acid.
From exylmexir.blob.core.windows.net
Lead Chloride Solubility In Hydrochloric Acid at Edward Burns blog Copper Chloride Reacts With Hydrochloric Acid you can think of this happening in two stages. First, you get copper(i) chloride formed: On the other hand, sodium is too reactive. copper is a very unreactive metal, and it does not react with hydrochloric acid. copper(i) chloride.is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. however, the situation. Copper Chloride Reacts With Hydrochloric Acid.
From study.com
Hydrogen Chloride vs. Hydrochloric Acid Formula, Properties Copper Chloride Reacts With Hydrochloric Acid however, the situation is slightly complicated because of the low solubility of $\ce{cucl}$ in dilute. copper is a very unreactive metal, and it does not react with hydrochloric acid. First, you get copper(i) chloride formed: copper oxide (cuo) (c u o) reacts with hydrochloric acid (h cl) (h c l) to produce copper (ii) chloride (cucl2) (c. Copper Chloride Reacts With Hydrochloric Acid.