What Is Mdd Vs Mdr . The mdr places more stringent requirements on notified bodies. While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical data. This table presents a summary of the provisions of some of the articles. For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical device regulation.” in european law, a directive is a set of guidelines setting minimum.
from www.johner-institut.de
While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. This table presents a summary of the provisions of some of the articles. This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical data. For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical device regulation.” in european law, a directive is a set of guidelines setting minimum. The mdr places more stringent requirements on notified bodies. Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,.
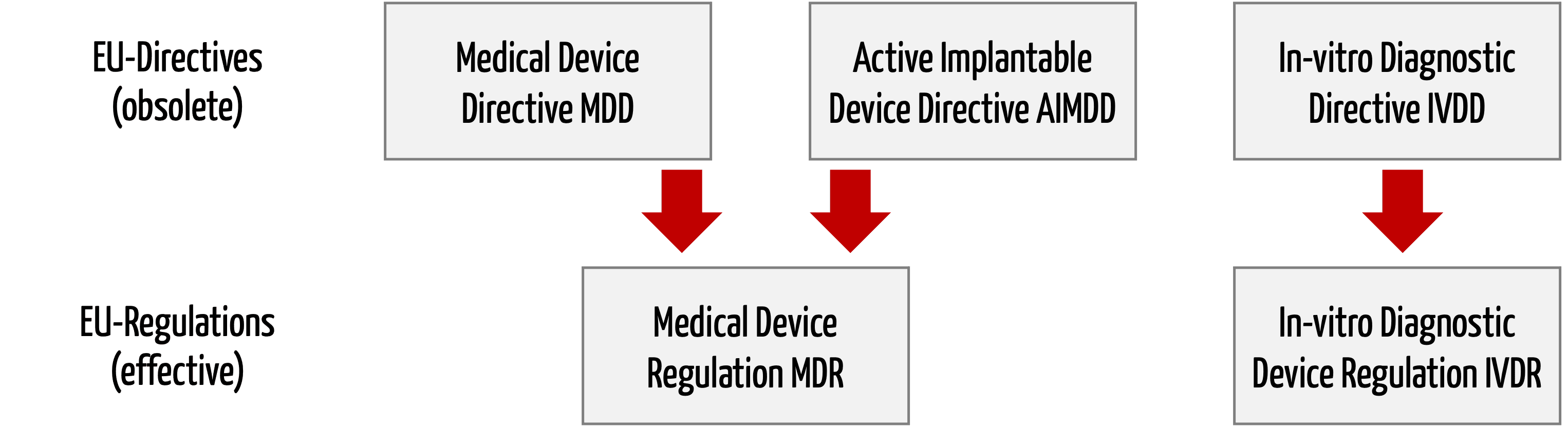
Unterschiede zwischen MDR und MDD
What Is Mdd Vs Mdr Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical data. Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical device regulation.” in european law, a directive is a set of guidelines setting minimum. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: The mdr places more stringent requirements on notified bodies. This table presents a summary of the provisions of some of the articles. This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe.
From www.operonstrategist.com
MDD to New MDR Classification of Medical Devices What Is Mdd Vs Mdr While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. While the mdd focused on getting a product to market, the mdr expands to consider. What Is Mdd Vs Mdr.
From www.slideserve.com
PPT MDR vs. MDD 13 Key Changes PowerPoint Presentation, free download What Is Mdd Vs Mdr Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. Learn the key differences between the new medical device regulation (mdr) and the medical devices. What Is Mdd Vs Mdr.
From tsquality.ch
MDR vs MDD QUICK COMPARISON TSQuality.ch What Is Mdd Vs Mdr This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. The mdr. What Is Mdd Vs Mdr.
From fyormmdbg.blob.core.windows.net
Mdd Vs Mdr Device Classification at Walter Franzen blog What Is Mdd Vs Mdr For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical device regulation.” in european law, a directive is a set of guidelines setting minimum. Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. This article will explore the difference between mdd. What Is Mdd Vs Mdr.
From gcpcentral.com
MDR VS MDD WHAT YOU NEED TO KNOW GCP Central What Is Mdd Vs Mdr Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. This article will explore the difference between mdd and mdr, provide you with. What Is Mdd Vs Mdr.
From intellisoft.io
EU Regulation Transitioning from the MDD to MDR What Is Mdd Vs Mdr This table presents a summary of the provisions of some of the articles. While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. The mdr places. What Is Mdd Vs Mdr.
From advisera.com
EU MDR vs. MDD Key differences [Infographic] What Is Mdd Vs Mdr For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical device regulation.” in european law, a directive is a set of guidelines setting minimum. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. While the mdd focused on getting a product to market, the. What Is Mdd Vs Mdr.
From clin-r.com
MDD vs MDR FAQ Clin R What Is Mdd Vs Mdr The mdr places more stringent requirements on notified bodies. This table presents a summary of the provisions of some of the articles. While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical device regulation.”. What Is Mdd Vs Mdr.
From tsquality.ch
MDR vs MDD QUICK COMPARISON TSQuality.ch What Is Mdd Vs Mdr This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: Understanding the key differences between mdd and mdr. What Is Mdd Vs Mdr.
From kobridgeconsulting.com
MDD vs MDR Understanding the difference Kobridge What Is Mdd Vs Mdr While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. The mdr places more stringent requirements on notified bodies. This table presents a summary of the provisions of some of the articles. The mdr requires a safety approach that is based on the entire product life cycle. What Is Mdd Vs Mdr.
From www.congress-intercultural.eu
EU MDR MDD Key Differences [Infographic], 58 OFF What Is Mdd Vs Mdr For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical device regulation.” in european law, a directive is a set of guidelines setting minimum. This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe.. What Is Mdd Vs Mdr.
From www.complianceteamllc.com
MDD Vs. MDR What’s the Difference? What Is Mdd Vs Mdr While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. The mdr places more stringent requirements on notified. What Is Mdd Vs Mdr.
From swarali-bhakre.medium.com
MDR vs. MDD 13 Key Changes. All Those involved in medical device… by What Is Mdd Vs Mdr Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical. What Is Mdd Vs Mdr.
From www.johner-institut.de
Unterschiede zwischen MDR und MDD What Is Mdd Vs Mdr While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. The mdr places more stringent requirements on notified bodies. Learn the key differences between the new medical device regulation. What Is Mdd Vs Mdr.
From advisera.com
What is EU MDR? Advisera What Is Mdd Vs Mdr The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical data. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. This article will explore the difference between mdd and mdr, provide you with an overview of what it. What Is Mdd Vs Mdr.
From operonstrategist.com
Medical Devices Directive (MDD) Related to MDR Classification and Their What Is Mdd Vs Mdr This table presents a summary of the provisions of some of the articles. The mdr places more stringent requirements on notified bodies. Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. While the mdd comprises 23 articles and 12 annexes over 60 pages, the. What Is Mdd Vs Mdr.
From www.regulatoryglobe.com
MDD vs MDR GapAssessment Tool What Is Mdd Vs Mdr This table presents a summary of the provisions of some of the articles. Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry.. What Is Mdd Vs Mdr.
From intellisoft.io
EU Regulation Transitioning from the MDD to MDR What Is Mdd Vs Mdr This table presents a summary of the provisions of some of the articles. The mdr places more stringent requirements on notified bodies. The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical data. While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and. What Is Mdd Vs Mdr.
From www.youtube.com
Raaj GPRAC lecture what is MDD and MDR17 differences YouTube What Is Mdd Vs Mdr While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. Learn the key differences between the new medical device regulation (mdr) and the medical devices. What Is Mdd Vs Mdr.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 59 OFF What Is Mdd Vs Mdr The mdr places more stringent requirements on notified bodies. This table presents a summary of the provisions of some of the articles. This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. Learn the key differences between. What Is Mdd Vs Mdr.
From www.mdr.guide
MDR Guidance Medical Device Regulatory Guide What Is Mdd Vs Mdr This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. For starters, “mdd” stands. What Is Mdd Vs Mdr.
From fyormmdbg.blob.core.windows.net
Mdd Vs Mdr Device Classification at Walter Franzen blog What Is Mdd Vs Mdr While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items. What Is Mdd Vs Mdr.
From fyormmdbg.blob.core.windows.net
Mdd Vs Mdr Device Classification at Walter Franzen blog What Is Mdd Vs Mdr This table presents a summary of the provisions of some of the articles. This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr. What Is Mdd Vs Mdr.
From www.greenlight.guru
MDD vs. MDR Gap Assessment Tool Free Download What Is Mdd Vs Mdr The mdr places more stringent requirements on notified bodies. This table presents a summary of the provisions of some of the articles. Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. The mdr requires a safety approach that is based on the entire product. What Is Mdd Vs Mdr.
From fyormmdbg.blob.core.windows.net
Mdd Vs Mdr Device Classification at Walter Franzen blog What Is Mdd Vs Mdr Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical. What Is Mdd Vs Mdr.
From www.bharatagritech.com
EU MDR MDD Key Differences [Infographic], 46 OFF What Is Mdd Vs Mdr The mdr places more stringent requirements on notified bodies. While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: This table presents a summary of the provisions of some of the articles. The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical. What Is Mdd Vs Mdr.
From www.zenarmor.com
What is Managed Detection and Response (MDR)? What Is Mdd Vs Mdr Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical device regulation.” in european law, a directive is a set of guidelines setting minimum. The mdr places more stringent requirements on notified bodies. Learn the. What Is Mdd Vs Mdr.
From www.differencebetween.net
Difference Between Adjustment Disorder and MDD Difference Between What Is Mdd Vs Mdr While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical data. While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over. What Is Mdd Vs Mdr.
From intellisoft.io
EU Regulation Transitioning from the MDD to MDR What Is Mdd Vs Mdr While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. The mdr requires a safety approach that is based on the entire product life cycle. What Is Mdd Vs Mdr.
From gidos.net
Gap Assessment MDD GiDoS What Is Mdd Vs Mdr The mdr places more stringent requirements on notified bodies. This table presents a summary of the provisions of some of the articles. This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. While the mdd focused on. What Is Mdd Vs Mdr.
From tsquality.ch
MDR vs MDD QUICK COMPARISON TSQuality.ch What Is Mdd Vs Mdr While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. For starters, “mdd” stands for “medical device directive,” and “mdr” stands for “medical device regulation.” in european law, a directive is a set of guidelines setting minimum. The mdr requires a safety approach that is based on. What Is Mdd Vs Mdr.
From www.youtube.com
MDD to MDR Transition Timeline YouTube What Is Mdd Vs Mdr This article will explore the difference between mdd and mdr, provide you with an overview of what it entails, and discuss how compliance can help your business to safely do business in europe. Understanding the key differences between mdd and mdr is crucial for manufacturers, regulatory professionals, and other stakeholders in the medical device industry. This table presents a summary. What Is Mdd Vs Mdr.
From www.scribd.com
Classification MDD Vs MDR PDF Tissue (Biology) Skin What Is Mdd Vs Mdr The mdr places more stringent requirements on notified bodies. While the mdd focused on getting a product to market, the mdr expands to consider the full product lifecycle: This table presents a summary of the provisions of some of the articles. The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical. What Is Mdd Vs Mdr.
From www.kapstonemedical.com
Three Key Differences Between MDD and MDR What Is Mdd Vs Mdr The mdr places more stringent requirements on notified bodies. The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical data. This table presents a summary of the provisions of some of the articles. This article will explore the difference between mdd and mdr, provide you with an overview of what it. What Is Mdd Vs Mdr.
From ndgcs.com
Transition from MDD to MDR Key Changes and Implications ND Global What Is Mdd Vs Mdr Learn the key differences between the new medical device regulation (mdr) and the medical devices direction (mdd), including items related to safety, performance, clinical data,. The mdr requires a safety approach that is based on the entire product life cycle and supported by clinical data. While the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has. What Is Mdd Vs Mdr.