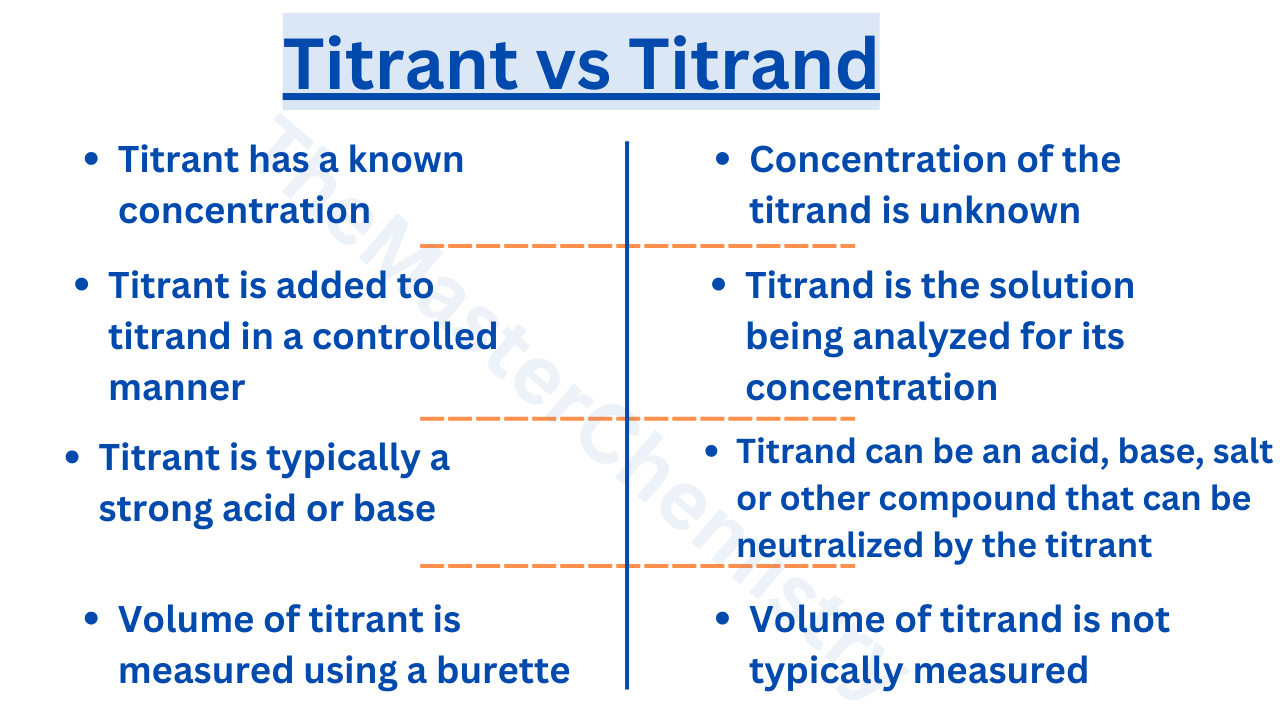
Titrate Definition And Titrant . titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. Titrant is a term used to describe the solution with a known concentration that is added to. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. the key difference between a titrant and a titrand is their role in titration.
from themasterchemistry.com
a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. the key difference between a titrant and a titrand is their role in titration. Titrant is a term used to describe the solution with a known concentration that is added to.
Titrant Vs. Titrand Understanding The Difference
Titrate Definition And Titrant Titrant is a term used to describe the solution with a known concentration that is added to. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. Titrant is a term used to describe the solution with a known concentration that is added to. the key difference between a titrant and a titrand is their role in titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.
From ar.inspiredpencil.com
Titration Diagram Titrate Definition And Titrant Titrant is a term used to describe the solution with a known concentration that is added to. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Titrate Definition And Titrant.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titrate Definition And Titrant titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titrant is a term used to describe the solution with a known concentration that is added to. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of. Titrate Definition And Titrant.
From es.slideshare.net
Tang 07 titrations 2 Titrate Definition And Titrant a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. It is used in quantitative analytical chemistry to determine. Titrate Definition And Titrant.
From themasterchemistry.com
Titrant Vs. Titrand Understanding The Difference Titrate Definition And Titrant titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration is the slow addition of one solution. Titrate Definition And Titrant.
From gioajmlyz.blob.core.windows.net
Titration Chemistry Research at Nicole Apple blog Titrate Definition And Titrant titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. Titrant is a term used to describe. Titrate Definition And Titrant.
From www.brand.de
Titration with the bottletop burette Titrette® Titrate Definition And Titrant It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. the key difference between a titrant and a titrand is their role in titration. Titrant is a term used to describe the solution with a known concentration that is added to. titration involves the gradual addition of a reagent of known concentration,. Titrate Definition And Titrant.
From www.microlit.com
An Advanced Guide to Titration Microlit Titrate Definition And Titrant titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration is the process in which one solution is added to another solution. Titrate Definition And Titrant.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Titrate Definition And Titrant the key difference between a titrant and a titrand is their role in titration. Titrant is a term used to describe the solution with a known concentration that is added to. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately. Titrate Definition And Titrant.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titrate Definition And Titrant titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. the key difference between a titrant and a titrand is their role in titration. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of. Titrate Definition And Titrant.
From thecontentauthority.com
Titration vs Titrant Differences And Uses For Each One Titrate Definition And Titrant titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Titrant is a term used to describe the solution with a known concentration that is added to. the key difference between. Titrate Definition And Titrant.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titrate Definition And Titrant titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. the key difference between a titrant and a titrand is their role in. Titrate Definition And Titrant.
From chimactiv.agroparistech.fr
Chimactiv Interactive numerical educational resources for the Titrate Definition And Titrant the key difference between a titrant and a titrand is their role in titration. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the process in which one solution is added to another solution such that it reacts under conditions in. Titrate Definition And Titrant.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Titrate Definition And Titrant titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titrant is a term used to describe the solution with a known concentration that is added to. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured. Titrate Definition And Titrant.
From www.askdifference.com
Titrant vs. Titrate — What’s the Difference? Titrate Definition And Titrant a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration, process of chemical analysis in which the quantity of some constituent of. Titrate Definition And Titrant.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titrate Definition And Titrant Titrant is a term used to describe the solution with a known concentration that is added to. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. the key. Titrate Definition And Titrant.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Titrate Definition And Titrant Titrant is a term used to describe the solution with a known concentration that is added to. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration, process of chemical analysis in which the quantity of some constituent of. Titrate Definition And Titrant.
From theedge.com.hk
Chemistry How To Titration The Edge Titrate Definition And Titrant Titrant is a term used to describe the solution with a known concentration that is added to. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration is the process. Titrate Definition And Titrant.
From www.youtube.com
Titration 01 Titrant and Titrand IIT, JEE & NEET YouTube Titrate Definition And Titrant a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the slow addition of one solution of a known concentration (called. Titrate Definition And Titrant.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titrate Definition And Titrant It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration involves the gradual addition of a reagent of known concentration, known as the. Titrate Definition And Titrant.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titrate Definition And Titrant titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. Titrant is a term used to describe the solution with a known concentration that is added to. titration is the slow addition of one solution of a known concentration (called. Titrate Definition And Titrant.
From www.slideshare.net
Acid base titration Titrate Definition And Titrant Titrant is a term used to describe the solution with a known concentration that is added to. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of one solution of a known concentration (called. Titrate Definition And Titrant.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titrate Definition And Titrant titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration is the slow addition of one solution of a known concentration (called a titrant). Titrate Definition And Titrant.
From worksheetcampusslewed.z21.web.core.windows.net
How To Do Titrations In Chemistry Titrate Definition And Titrant It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments. Titrate Definition And Titrant.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titrate Definition And Titrant titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titrant is a term used to describe the solution with a known concentration that is added to. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titrate Definition And Titrant.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Titrate Definition And Titrant titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. It is used in quantitative analytical chemistry to determine an unknown concentration of an. Titrate Definition And Titrant.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Titrate Definition And Titrant It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration is the process in which one solution is added to another solution such that it reacts under conditions in which. Titrate Definition And Titrant.
From byjus.com
What is to titrant and titrat Titrate Definition And Titrant titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of. Titrate Definition And Titrant.
From hxesyopwi.blob.core.windows.net
Titration Definition In Education at Alexandra Bradford blog Titrate Definition And Titrant a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. It is used in quantitative analytical chemistry to determine an unknown concentration of an. Titrate Definition And Titrant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Titrate Definition And Titrant titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. Titrant is a term used to describe the solution. Titrate Definition And Titrant.
From school.careers360.com
types of titration Overview, Structure, Properties & Uses Titrate Definition And Titrant a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titrant is a term used to describe the solution with a known concentration that. Titrate Definition And Titrant.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titrate Definition And Titrant titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. the key difference between a titrant and a titrand is their role in titration. Titrant is a term used to describe the solution with a known concentration that is added. Titrate Definition And Titrant.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145156 Titrate Definition And Titrant titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. the key difference between a titrant and a titrand is their role in. Titrate Definition And Titrant.
From www.youtube.com
Titration Definition, steps and formula YouTube Titrate Definition And Titrant the key difference between a titrant and a titrand is their role in titration. Titrant is a term used to describe the solution with a known concentration that is added to. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately. Titrate Definition And Titrant.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titrate Definition And Titrant Titrant is a term used to describe the solution with a known concentration that is added to. the key difference between a titrant and a titrand is their role in titration. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. It is used in. Titrate Definition And Titrant.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry / PHC 213 PowerPoint Titrate Definition And Titrant titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is defined as ‘the process of determining the quantity. Titrate Definition And Titrant.