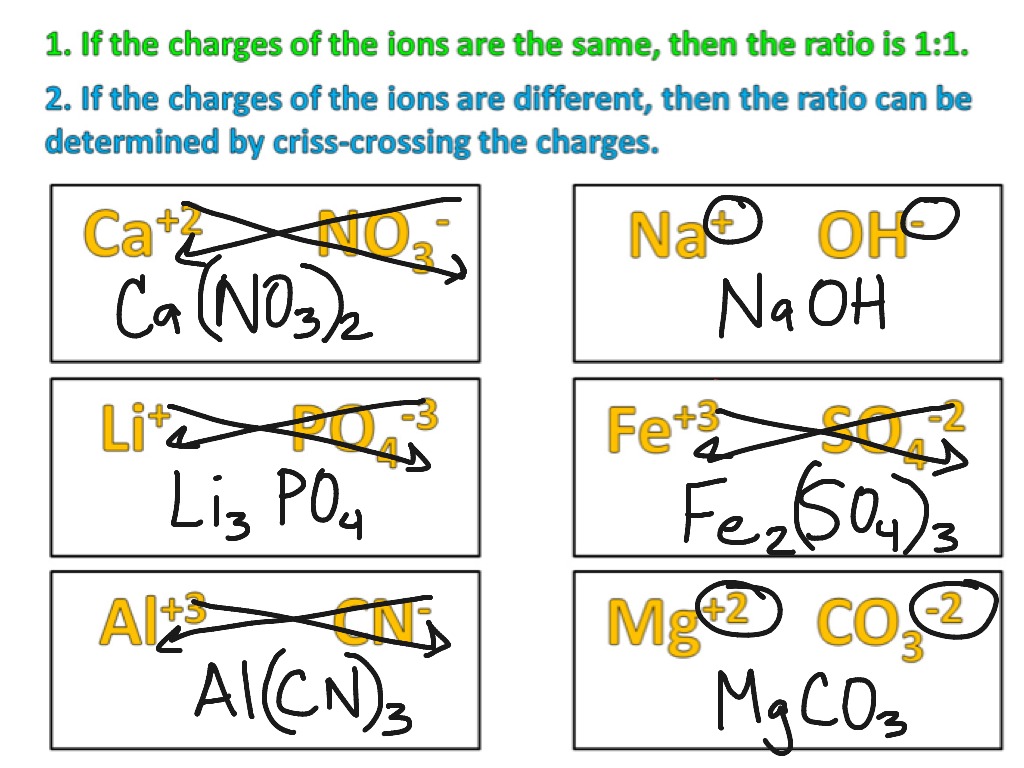
Zinc Acetate Cation And Anion . Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. The active moiety in zinc acetate is zinc cation. Since the compound needs to be neutral,. The lighter group 3a metals (aluminum, galium and. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. A white solid, this compound is composed of zinc cations and acetate anions. This is the most common method of. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological.
from socratic.org
This is the most common method of. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The lighter group 3a metals (aluminum, galium and. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. A white solid, this compound is composed of zinc cations and acetate anions. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. Since the compound needs to be neutral,. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. The active moiety in zinc acetate is zinc cation.
How do you write the formula for zinc acetate? Socratic
Zinc Acetate Cation And Anion Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. A white solid, this compound is composed of zinc cations and acetate anions. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. The lighter group 3a metals (aluminum, galium and. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Since the compound needs to be neutral,. The active moiety in zinc acetate is zinc cation. This is the most common method of. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological.
From www.researchgate.net
(PDF) Synergistic Effect of Cation and Anion for LowTemperature Zinc Acetate Cation And Anion Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. A white solid, this compound is composed. Zinc Acetate Cation And Anion.
From pubs.rsc.org
The separation of zinc from some other elements by means of anion Zinc Acetate Cation And Anion This is the most common method of. A white solid, this compound is composed of zinc cations and acetate anions. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Since the compound needs to be neutral,. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are. Zinc Acetate Cation And Anion.
From vanspa.vn
Zinc Acetate Zinc Acetate Cation And Anion Since the compound needs to be neutral,. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. A white solid, this compound is composed of zinc cations and acetate anions. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Zinc forms. Zinc Acetate Cation And Anion.
From slideplayer.com
Chemical Nomenclature ppt download Zinc Acetate Cation And Anion This is the most common method of. The active moiety in zinc acetate is zinc cation. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. A white solid, this compound is composed of zinc cations and acetate anions. Zinc and cadmium (group 2b) form [+2] cations like the group 1b. Zinc Acetate Cation And Anion.
From www.slideserve.com
PPT Chemical Bonds IONIC BONDING PowerPoint Presentation, free Zinc Acetate Cation And Anion Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Since the compound needs to be neutral,. The lighter group 3a metals (aluminum, galium and. A white solid, this compound is composed of zinc cations and. Zinc Acetate Cation And Anion.
From www.youtube.com
Cation Test Zinc Ions YouTube Zinc Acetate Cation And Anion Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. The lighter group 3a metals (aluminum, galium and. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Since the compound needs to be neutral,. Regardless of the ligand, zinc blocks the intestinal absorption of copper. Zinc Acetate Cation And Anion.
From www.dreamstime.com
Skeletal Formula of Acetate Anion, Chemical Structure. Stock Vector Zinc Acetate Cation And Anion Since the compound needs to be neutral,. The lighter group 3a metals (aluminum, galium and. The active moiety in zinc acetate is zinc cation. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions. Zinc Acetate Cation And Anion.
From stock.adobe.com
Zinc Acetate Properties and Chemical Compound Structure Vector Design Zinc Acetate Cation And Anion Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. It possesses the chemical formula c 4. Zinc Acetate Cation And Anion.
From www.researchgate.net
Effect of anion on the morphology and particles sizes of sample; (a Zinc Acetate Cation And Anion The active moiety in zinc acetate is zinc cation. This is the most common method of. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. A white solid, this compound is. Zinc Acetate Cation And Anion.
From socratic.org
How do you write the formula for zinc acetate? Socratic Zinc Acetate Cation And Anion Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. This is the most common method of. Since the compound needs to be neutral,. It possesses the chemical formula c 4 h. Zinc Acetate Cation And Anion.
From pubs.acs.org
Understanding and Expanding Zinc Cation/Amine Frustrated Lewis Pair Zinc Acetate Cation And Anion Since the compound needs to be neutral,. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. The. Zinc Acetate Cation And Anion.
From slideplayer.com
Predicting Products Chemical Reactions. ppt download Zinc Acetate Cation And Anion It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. A white solid, this compound is composed of zinc cations and acetate anions. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. The active moiety in zinc acetate is zinc cation. The lighter group 3a metals. Zinc Acetate Cation And Anion.
From chemistry-europe.onlinelibrary.wiley.com
Cation and Anion Modulation Strategies toward Ideal Zinc Artificial Zinc Acetate Cation And Anion Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The active moiety in zinc acetate is zinc cation. The lighter group 3a metals (aluminum, galium and. This is the most common method of. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Acetate, carbonate,. Zinc Acetate Cation And Anion.
From www.researchgate.net
(a) The mechanism for the reaction of zinc acetate and DEA in Ethanol Zinc Acetate Cation And Anion Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The lighter group 3a metals (aluminum, galium and. A white solid, this compound is composed of zinc cations and acetate anions. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. The active moiety in zinc. Zinc Acetate Cation And Anion.
From www.numerade.com
SOLVED Ionic Compound Formulas For each of the ionic compounds below Zinc Acetate Cation And Anion It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. This is the most common method of. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological.. Zinc Acetate Cation And Anion.
From achs-prod.acs.org
Chemical Equilibrium of Zinc Acetate Complexes in Ethanol Solution. A Zinc Acetate Cation And Anion Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Since the compound needs to be neutral,. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously.. Zinc Acetate Cation And Anion.
From diferenciando.com
Diferencia entre aniónes y catiónes (con nomenclatura y ejemplos Zinc Acetate Cation And Anion Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. The lighter group 3a metals (aluminum, galium and. A white solid, this compound is composed of zinc cations and acetate anions. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. The. Zinc Acetate Cation And Anion.
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell Zinc Acetate Cation And Anion Since the compound needs to be neutral,. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. This is the most common method of. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. Zinc and cadmium (group 2b) form [+2] cations like. Zinc Acetate Cation And Anion.
From www.researchgate.net
Comparison of the positions of the acetate ion and the zinc ions in the Zinc Acetate Cation And Anion Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. The lighter group 3a metals (aluminum, galium and. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. A white solid, this compound is composed of zinc cations and acetate anions. Zinc forms 2+ cations, with. Zinc Acetate Cation And Anion.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Zinc Acetate Cation And Anion The active moiety in zinc acetate is zinc cation. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. The lighter group 3a metals (aluminum, galium and. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. It possesses the chemical formula. Zinc Acetate Cation And Anion.
From www.dreamstime.com
Skeletal Formula of Acetate Anion, Chemical Structure Stock Vector Zinc Acetate Cation And Anion Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. This is the most common method of. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The active moiety in. Zinc Acetate Cation And Anion.
From www.vectorstock.com
Zinc acetate formula chemical Royalty Free Vector Image Zinc Acetate Cation And Anion The lighter group 3a metals (aluminum, galium and. This is the most common method of. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. The active moiety in zinc acetate is zinc cation. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula. Zinc Acetate Cation And Anion.
From www.youtube.com
How to Write the Formula for Zinc acetate YouTube Zinc Acetate Cation And Anion This is the most common method of. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. A white solid, this compound is composed of zinc cations and acetate anions. Acetate, carbonate, and chloride are ligands with. Zinc Acetate Cation And Anion.
From www.researchgate.net
Achieving Synergetic Anion‐Cation Redox Chemistry in Freestanding Zinc Acetate Cation And Anion It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. The active moiety in zinc acetate is zinc cation. Since the compound needs to be neutral,. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions,. Zinc Acetate Cation And Anion.
From www.dreamstime.com
Zinc Acetate is a Molecular Chemical Formula. Zinc Infographics. Vector Zinc Acetate Cation And Anion Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. A white solid, this compound is composed of zinc cations and acetate anions. Since the compound needs to be neutral,. Regardless of the ligand, zinc blocks the. Zinc Acetate Cation And Anion.
From slideplayer.com
Chemical Bonds. ppt download Zinc Acetate Cation And Anion Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Since the compound needs to be neutral,.. Zinc Acetate Cation And Anion.
From issuu.com
Zinc AcetateFormula And Uses by Amizara Chemicals Issuu Zinc Acetate Cation And Anion Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. Since the compound needs to be neutral,. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. The active moiety in zinc acetate is zinc cation. The lighter group 3a metals (aluminum, galium. Zinc Acetate Cation And Anion.
From www.numerade.com
SOLVED Provide an IUPAC name or formula Write the cation Or anion if Zinc Acetate Cation And Anion Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. The lighter group 3a metals (aluminum, galium and. This is the most common method of. The active moiety in zinc acetate is zinc cation. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula. Zinc Acetate Cation And Anion.
From www.chegg.com
Solved Complete the following table Cation Formula Anion Zinc Acetate Cation And Anion Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. The lighter group 3a metals (aluminum, galium and. A white solid,. Zinc Acetate Cation And Anion.
From www.researchgate.net
Geometric parameters of transition state of zinc acetatecatalyzed Zinc Acetate Cation And Anion Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. This is the most common method of. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Zinc. Zinc Acetate Cation And Anion.
From www.numerade.com
SOLVED Ionic Compound Formulas For each of the ionic compounds below Zinc Acetate Cation And Anion Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Since the compound needs to be neutral,. The active moiety in zinc acetate is zinc cation. This is the most common method of. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Zinc and cadmium. Zinc Acetate Cation And Anion.
From www.researchgate.net
Structures of cations and anions, names and abbreviations. Download Zinc Acetate Cation And Anion This is the most common method of. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. Acetate,. Zinc Acetate Cation And Anion.
From www.numerade.com
SOLVED PART I Ions and Formulas of Ionic Compounds Write the symbol Zinc Acetate Cation And Anion The lighter group 3a metals (aluminum, galium and. Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological.. Zinc Acetate Cation And Anion.
From www.chegg.com
Solved TABLE 5.47 Name Compound Cation Anion 1. zinc Zinc Acetate Cation And Anion It possesses the chemical formula c 4 h 6 o 4 zn and belongs to. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. This is the most common method of. Since the compound needs to be neutral,. A white solid, this compound is composed of zinc cations and acetate. Zinc Acetate Cation And Anion.
From www.youtube.com
Is ZnCl2 (Zinc chloride) Ionic or Covalent? YouTube Zinc Acetate Cation And Anion Zinc forms 2+ cations, with the formula #zn^(2+)# and acetate is a polyatomic anion with the formula #c_2h_3o_2^−#. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Acetate, carbonate, and chloride are ligands with intermediate strength for coordinating with zinc ions, and some anions are biological. Regardless of the ligand,. Zinc Acetate Cation And Anion.