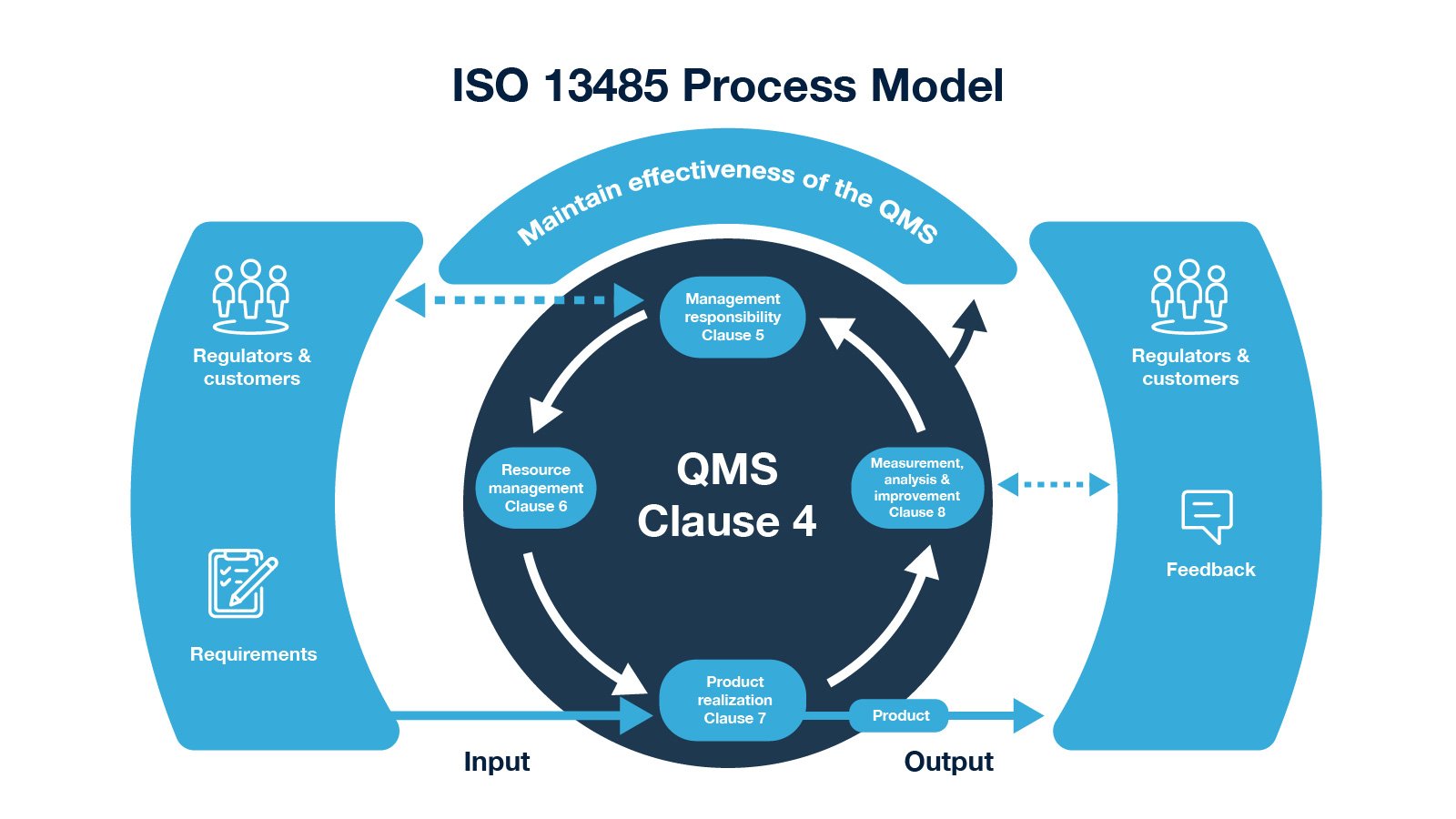
Medical Device Definition Iso 13485 . Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Every medical device type or device family must have a medical device file. This standard is specific to medical. Iso 13485 requires the contents of a medical. Iso 13485 requires a medical device file since the 2016 edition. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry.
from blog.sierralabs.com
Iso 13485 requires the contents of a medical. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Iso 13485 requires a medical device file since the 2016 edition. En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. This standard is specific to medical. Every medical device type or device family must have a medical device file.
ISO 13485 Regulatory Requirements on Medical Devices
Medical Device Definition Iso 13485 Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Iso 13485 requires the contents of a medical. Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. Iso 13485 requires a medical device file since the 2016 edition. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. This standard is specific to medical. Every medical device type or device family must have a medical device file.
From gxp-training.com
Medical Devices ISO 13485 Internal Auditor Course & Certification Medical Device Definition Iso 13485 Iso 13485 requires the contents of a medical. En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Every medical device type or device family must have a medical device file. Iso 13485 requires a medical device file since the 2016 edition. The aim is to ensure that manufacturers create (and. Medical Device Definition Iso 13485.
From www.exportersindia.com
Services ISO 134852016 Medical Device Certification Services from Medical Device Definition Iso 13485 En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where. Medical Device Definition Iso 13485.
From lightnovelfit.com
What Should An ISO 13485 Medical Device Quality Manual Look Like? Medical Device Definition Iso 13485 Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Every medical device type or device family must have a medical device file.. Medical Device Definition Iso 13485.
From www.scribd.com
Iso 13485 Medical Devices 2016 Medical Device Iso 9000 Medical Device Definition Iso 13485 Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Iso 13485 requires the contents of a medical. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Iso 13485 requires a medical device file since the 2016. Medical Device Definition Iso 13485.
From www.youtube.com
What is ISO 13485 for medical devices? YouTube Medical Device Definition Iso 13485 En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to.. Medical Device Definition Iso 13485.
From www.vectorstock.com
Iso 13485 stamp sign medical devices quality Vector Image Medical Device Definition Iso 13485 The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. Iso 13485 requires the contents of a medical. This international standard specifies requirements for a quality management system that. Medical Device Definition Iso 13485.
From kb-intl.com
ISO 134852016 KB International Medical Device Definition Iso 13485 Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. En iso. Medical Device Definition Iso 13485.
From www.youtube.com
ISO 13485 QMS for Medical Devices Standard Basic Introduction YouTube Medical Device Definition Iso 13485 Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate. Medical Device Definition Iso 13485.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Medical Device Definition Iso 13485 Iso 13485 requires a medical device file since the 2016 edition. En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Iso 13485 is an international standard that outlines the requirements for a. Medical Device Definition Iso 13485.
From www.pinterest.com
Overview of ISO 13485 Quality Management Standard for Medical Devices Medical Device Definition Iso 13485 This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Iso 13485 requires the contents of a medical. Every medical device type or device family must have a medical. Medical Device Definition Iso 13485.
From www.quality-assurance.com
ISO 13485 standard for medical devices How Effective it is? Medical Device Definition Iso 13485 The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. Iso 13485 requires a medical device file since the 2016 edition. Iso 13485 requires the contents of a medical. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. En iso 13485 is revised so that. Medical Device Definition Iso 13485.
From safetybro.weebly.com
Iso 13485 latest version safetybro Medical Device Definition Iso 13485 En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or. Medical Device Definition Iso 13485.
From www.4cpl.com
ISO 13485 Enhancing Medical Device Quality & Compliance Medical Device Definition Iso 13485 Iso 13485 requires the contents of a medical. Every medical device type or device family must have a medical device file. Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. This international standard specifies requirements for a quality management system that can be used by an organization involved in. Medical Device Definition Iso 13485.
From in.pinterest.com
Who need iso 13485 certification. Iso 13485, Iso, Cert Medical Device Definition Iso 13485 Iso 13485 requires a medical device file since the 2016 edition. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. Every medical device type or device family must have a medical device file. Iso 13485 is an international standard that outlines the requirements for. Medical Device Definition Iso 13485.
From cliniexperts.com
ISO 13485 Medical Devices Certification Medical Device ISO Standards Medical Device Definition Iso 13485 This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to.. Medical Device Definition Iso 13485.
From glenviewgroupinc.weebly.com
ISO 13485 Quality Management System for Medical Devices Certification Medical Device Definition Iso 13485 Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. Every medical. Medical Device Definition Iso 13485.
From www.quality-assurance.com
Why Is ISO 13485 Standard for Medical Devices Popular Medical Device Definition Iso 13485 Every medical device type or device family must have a medical device file. Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. This standard is specific to medical. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. En iso 13485 is revised. Medical Device Definition Iso 13485.
From ramtechno.com
ISO 13485 for Medical Devices RAM Technologies Medical Device Definition Iso 13485 Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Every medical device type or device family must have a medical device file. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. This standard is specific to medical. Iso 13485 requires. Medical Device Definition Iso 13485.
From apanakarobar.blogspot.com
ISO 13485 Certification ( Medical Devices Quality Management system Medical Device Definition Iso 13485 The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. Iso 13485 requires a medical device file since the 2016 edition. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Iso 13485 requires the contents of a medical. Iso 13485 is. Medical Device Definition Iso 13485.
From ossmideast.com
Medical device(QMS)ISO 13485 OSS Middle East Certification Medical Device Definition Iso 13485 Iso 13485 requires a medical device file since the 2016 edition. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. En iso 13485 is revised so that it harmonizes. Medical Device Definition Iso 13485.
From www.gqsindia.com
Is ISO 13485 Certification mandatory for medical devices ? ISO 22000 Medical Device Definition Iso 13485 Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Every medical device type or device family must have a medical device file. This international standard specifies requirements for a quality management. Medical Device Definition Iso 13485.
From acc-co.com
ISO 13485 Medical Devices Quality Management System Trainings ACC Medical Device Definition Iso 13485 This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. This standard is specific to medical. Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Iso 13485:2016 specifies requirements for a quality management system where an organization. Medical Device Definition Iso 13485.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Medical Device Definition Iso 13485 This standard is specific to medical. Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. Iso 13485 requires a medical device file since the 2016 edition. Iso 13485:2016 specifies requirements for a quality. Medical Device Definition Iso 13485.
From www.pinterest.com
PPT ISO 134852016 (Medical Devices QMS) Awareness Training (67 Medical Device Definition Iso 13485 This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. Iso 13485 is an international standard that outlines the requirements for a quality. Medical Device Definition Iso 13485.
From www.qacqatar.com
ISO 13485 Medical Devices Quality Management Systems QGOS ISO Medical Device Definition Iso 13485 Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Every medical device type or device family must have a medical device file. This standard is specific to medical. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Iso. Medical Device Definition Iso 13485.
From www.indiamart.com
ISO 134852016 Medical Device Quality Management Certification Service Medical Device Definition Iso 13485 Every medical device type or device family must have a medical device file. Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. The aim is to ensure that manufacturers create (and. Medical Device Definition Iso 13485.
From www.tpsearchtool.com
Iso 13485 Starter Kit The Qms For Your Medical Device Images Medical Device Definition Iso 13485 Every medical device type or device family must have a medical device file. This standard is specific to medical. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: This international standard specifies requirements for. Medical Device Definition Iso 13485.
From www.slideserve.com
PPT What is the importance of ISO 13485 for a medical device Medical Device Definition Iso 13485 This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Iso 13485 requires a medical device file since the 2016 edition. This standard is specific to medical. Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where. Medical Device Definition Iso 13485.
From www.4cpl.com
ISO 134852016 Technical Quality Management for Medical Devices Medical Device Definition Iso 13485 Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. This standard is specific to medical. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. The aim is to ensure that manufacturers. Medical Device Definition Iso 13485.
From www.exsolutiongroup.com
ISO 134852003 Quality Management System for Medical Devices QMS for Medical Device Definition Iso 13485 En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Iso 13485 requires a medical device file since the 2016 edition. The aim is to ensure that manufacturers create (and maintain) all documents required to demonstrate compliance. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to. Medical Device Definition Iso 13485.
From seobwhmseo.weebly.com
Medical device iso 13485 seobwhmseo Medical Device Definition Iso 13485 Iso 13485 requires the contents of a medical. Every medical device type or device family must have a medical device file. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. Iso. Medical Device Definition Iso 13485.
From sworldvica.weebly.com
Iso 13485 medical devices sworldvica Medical Device Definition Iso 13485 This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Iso 13485 requires the contents of a medical. En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: This standard is specific to medical. Iso 13485 requires a medical. Medical Device Definition Iso 13485.
From www.iso-saudi.net
ISO 13485 Medical devices Quality management systems requirements Medical Device Definition Iso 13485 Iso 13485:2016 medical devices — quality management systems — requirements for regulatory purposes specifies requirements for a quality management system where an organization needs to. Every medical device type or device family must have a medical device file. Iso 13485 requires a medical device file since the 2016 edition. Iso 13485:2016 specifies requirements for a quality management system where an. Medical Device Definition Iso 13485.
From www.tradeindia.com
Iso 13485 Qms For Medical Device Industry at Best Price in Noida Medical Device Definition Iso 13485 Iso 13485 is an international standard that outlines the requirements for a quality management system in the medical device industry. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. Iso 13485 requires the contents of a medical. Iso 13485:2016 specifies requirements for a quality management system where. Medical Device Definition Iso 13485.
From www.iso.org
ISO 13485 Medical devices Medical Device Definition Iso 13485 En iso 13485 is revised so that it harmonizes with the three european directives associated with the medical sector: Iso 13485 requires a medical device file since the 2016 edition. This international standard specifies requirements for a quality management system that can be used by an organization involved in one or more. This standard is specific to medical. Iso 13485. Medical Device Definition Iso 13485.