How Chemistry Indicators Work . A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance in various chemical contexts. Indicators are substances that change color in response to a change in ph. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. The indicator changes color when it reaches a critical range of values. The following equilibrium exists for the indicator: They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Indicators are substances whose solutions change color due to changes in ph. Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec). This could be a color change, precipitate. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Used to determine the endpoint of a titration. Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases.
from www.slideserve.com
Indicators are substances whose solutions change color due to changes in ph. Indicators are substances that change color in response to a change in ph. The following equilibrium exists for the indicator: Used to determine the endpoint of a titration. The indicator changes color when it reaches a critical range of values. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This could be a color change, precipitate. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases.
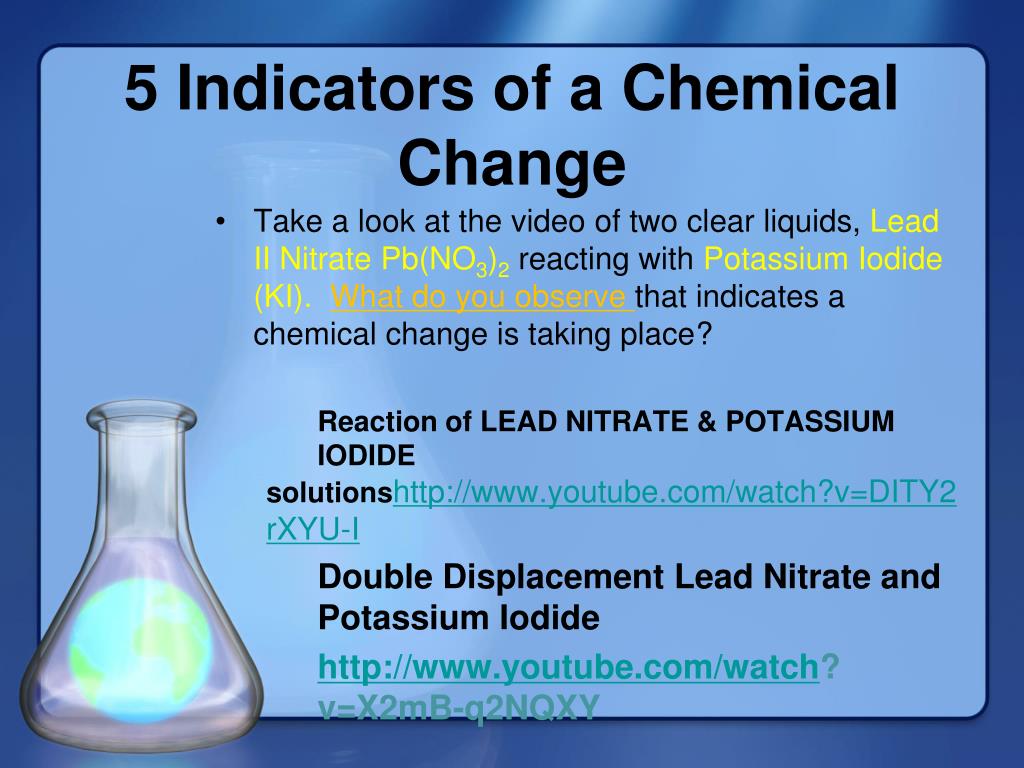
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free
How Chemistry Indicators Work Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec). The indicator changes color when it reaches a critical range of values. Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Indicators are substances that change color in response to a change in ph. Used to determine the endpoint of a titration. Indicators are substances whose solutions change color due to changes in ph. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. This could be a color change, precipitate. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance in various chemical contexts. The following equilibrium exists for the indicator:
From www.scribd.com
Indicators Titration Chemistry How Chemistry Indicators Work This could be a color change, precipitate. Indicators are substances that change color in response to a change in ph. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec). They are usually weak acids. How Chemistry Indicators Work.
From www.slideserve.com
PPT Chapter 16 AcidBase Equilibria PowerPoint Presentation, free How Chemistry Indicators Work Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. The following equilibrium exists for the indicator: Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec). They. How Chemistry Indicators Work.
From www.youtube.com
Chemistry 12 Lesson 22 Indicators YouTube How Chemistry Indicators Work Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. This could be a color change, precipitate. An indicator is a weak acid that ionizes within. How Chemistry Indicators Work.
From knowledge.carolina.com
AcidBase Indicators Carolina Knowledge Center How Chemistry Indicators Work Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec). Used to determine the endpoint of a titration. This could be a color change, precipitate. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. This article provides a comprehensive insight into chemical indicators, discussing. How Chemistry Indicators Work.
From nigerianscholars.com
AcidBase Indicators AcidBase Equilibria How Chemistry Indicators Work Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. Used to determine the endpoint of a titration. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec).. How Chemistry Indicators Work.
From www.pw.live
Indicators in chemistry Types of Indicators in chemistry Physics Wallah How Chemistry Indicators Work This could be a color change, precipitate. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. An indicator is a weak acid that ionizes within a known ph range, usually about 2. How Chemistry Indicators Work.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic How Chemistry Indicators Work Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. This could be a color change, precipitate. They are usually weak acids or bases, but their conjugate base or acid forms have different. How Chemistry Indicators Work.
From fphoto.photoshelter.com
science chemistry acid base universal indicator Fundamental How Chemistry Indicators Work This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance in various chemical contexts. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. How Chemistry Indicators Work.
From www.pw.live
Indicators in chemistry Types of Indicators in chemistry Physics Wallah How Chemistry Indicators Work The following equilibrium exists for the indicator: An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance in various chemical contexts. Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids. How Chemistry Indicators Work.
From www.youtube.com
IGCSE Chemistry Titration and indicators method question YouTube How Chemistry Indicators Work They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The indicator changes color when it reaches a critical range of values. Indicators are substances that change color in response to a change in ph. This could be a color change, precipitate. Indicators are substances whose. How Chemistry Indicators Work.
From www.slideserve.com
PPT Equilibrium Part 2 Chemistry 2 PowerPoint Presentation, free How Chemistry Indicators Work A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Indicators are substances that change color in response to a change in ph. Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. This could be a color change, precipitate. They are usually weak acids. How Chemistry Indicators Work.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive How Chemistry Indicators Work Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec). The following equilibrium exists for the. How Chemistry Indicators Work.
From byjus.com
Study The pH Change In The Titration Of A Strong Base Using Universal How Chemistry Indicators Work Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption. How Chemistry Indicators Work.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free How Chemistry Indicators Work This could be a color change, precipitate. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in. How Chemistry Indicators Work.
From www.youtube.com
Trick to Memorize Colours and pH of Indicators Organic Chemistry How Chemistry Indicators Work This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance in various chemical contexts. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Learn how chemicals. How Chemistry Indicators Work.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Chemistry Indicators Work The indicator changes color when it reaches a critical range of values. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. The following equilibrium exists for the indicator: They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption. How Chemistry Indicators Work.
From ccschemistry.blogspot.com
Cowbridge Chemistry Department Indicators Colours and pH ranges How Chemistry Indicators Work Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec). Indicators are substances that change color. How Chemistry Indicators Work.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Chemistry Indicators Work Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Indicators are substances that change color in response to a change in ph. This could be a color change, precipitate. A chemical indicator is a substance that undergoes a distinct observable change when. How Chemistry Indicators Work.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free How Chemistry Indicators Work A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. The following equilibrium exists for the indicator: This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance in various chemical contexts. Chemical indicator, any substance that gives a visible sign, usually by a color change, of. How Chemistry Indicators Work.
From www.youtube.com
WCLN Indicators How They Work Chemistry YouTube How Chemistry Indicators Work Used to determine the endpoint of a titration. The indicator changes color when it reaches a critical range of values. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their. How Chemistry Indicators Work.
From www.youtube.com
Chemistry ( indicators ) YouTube How Chemistry Indicators Work They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The indicator changes color when it reaches a critical range of values. Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. An indicator is a weak acid that. How Chemistry Indicators Work.
From materialcampuscogar.z13.web.core.windows.net
List Of Acid Base Indicators How Chemistry Indicators Work Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. The following equilibrium exists for the indicator: Indicators are substances whose solutions change color due to changes in ph. An indicator is a. How Chemistry Indicators Work.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Chemistry Indicators Work Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. This could be a color change, precipitate. This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance in various chemical contexts. They are usually weak acids or. How Chemistry Indicators Work.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry How Chemistry Indicators Work An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This could be a color change, precipitate. The indicator changes color when it reaches a critical range of values. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. How Chemistry Indicators Work.
From slideplayer.com
How simple indicators work. ppt download How Chemistry Indicators Work The indicator changes color when it reaches a critical range of values. Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. This could be a color change, precipitate. They are usually weak acids or. How Chemistry Indicators Work.
From www.youtube.com
3. Indicators and their uses (HSC chemistry) YouTube How Chemistry Indicators Work An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Indicators are substances that change color in response to a change in ph. Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec). They are usually weak acids or bases, but their conjugate base or. How Chemistry Indicators Work.
From www.youtube.com
Universal Indicator Acids, Bases and Salts Class 10 Chemistry How Chemistry Indicators Work They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Indicators are substances that change color in response to a change in ph. Chemical indicator, any substance that. How Chemistry Indicators Work.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ How Chemistry Indicators Work Indicators are substances that change color in response to a change in ph. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. The following equilibrium exists for the indicator: Chemical indicators play a crucial role in chemistry, particularly in understanding the properties. How Chemistry Indicators Work.
From chem.libretexts.org
3.3 AcidBase Indicators Chemistry LibreTexts How Chemistry Indicators Work Indicators are substances that change color in response to a change in ph. The indicator changes color when it reaches a critical range of values. This could be a color change, precipitate. The following equilibrium exists for the indicator: This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance in various chemical contexts. A. How Chemistry Indicators Work.
From www.compoundchem.com
The Colours & Chemistry of pH Indicators Compound Interest How Chemistry Indicators Work The following equilibrium exists for the indicator: Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance. How Chemistry Indicators Work.
From www.youtube.com
What Are Indicators & How Do We Use Them? Chemical Tests Chemistry How Chemistry Indicators Work Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species. How Chemistry Indicators Work.
From www.youtube.com
How pH Indicator Works // HSC Chemistry YouTube How Chemistry Indicators Work Used to determine the endpoint of a titration. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This could be a color change, precipitate.. How Chemistry Indicators Work.
From www.freepik.com
Premium Vector Chemical reaction indicators. How Chemistry Indicators Work Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. This could be a color change, precipitate. The following equilibrium exists for the indicator: They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Chemical indicator, any substance that. How Chemistry Indicators Work.
From bceweb.org
Chemistry Indicators Chart A Visual Reference of Charts Chart Master How Chemistry Indicators Work The indicator changes color when it reaches a critical range of values. Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Indicators are substances that change color in. How Chemistry Indicators Work.
From www.slideserve.com
PPT Equilibrium Part 2 Chemistry 2 PowerPoint Presentation, free How Chemistry Indicators Work Learn how chemicals are classified using the ph scale and indicators with bbc bitesize gcse chemistry (wjec). Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its. How Chemistry Indicators Work.