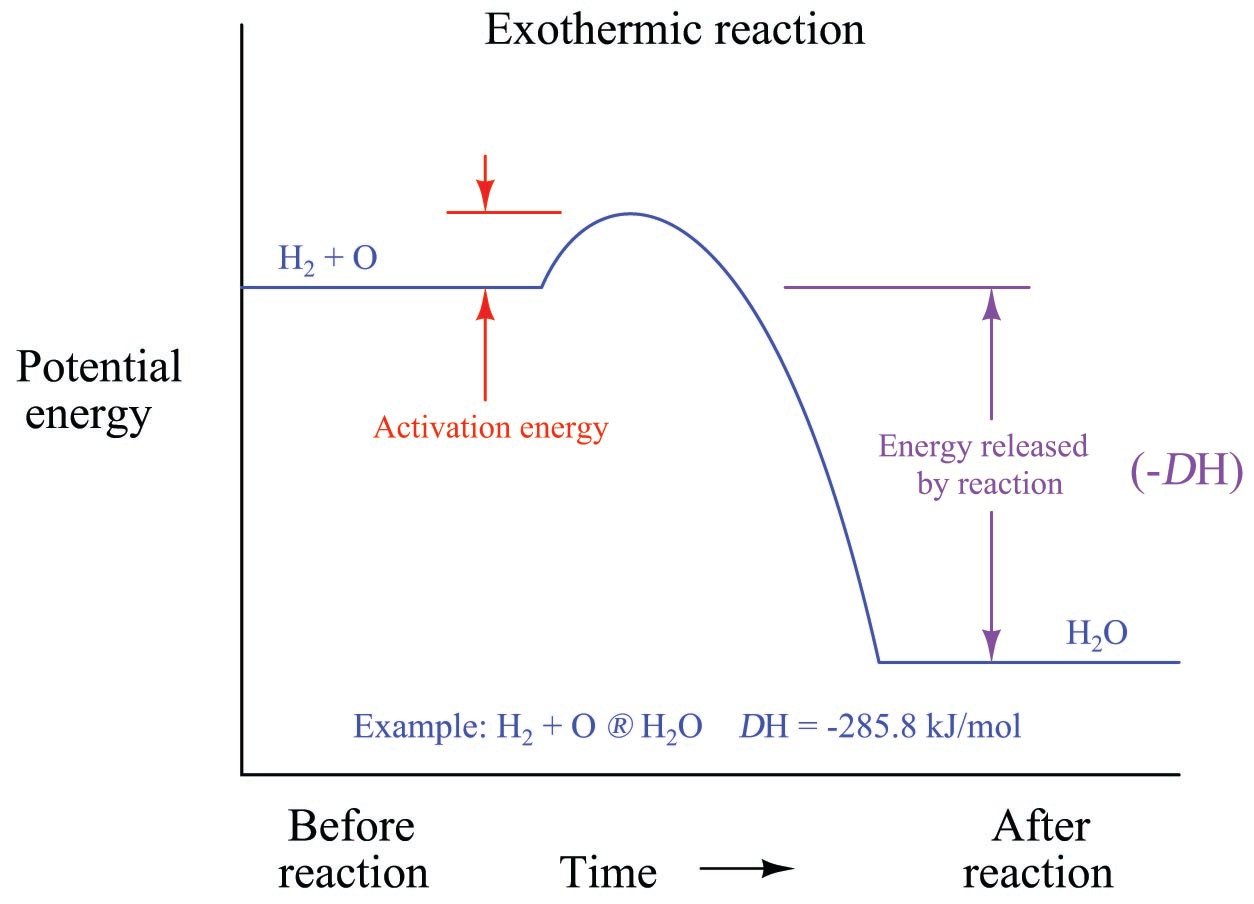
What Is A Reaction That Releases Energy . endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. When methane gas is combusted, heat is released, making the reaction exothermic. reactions that release energy: when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. Respiration is the chemical change. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a.
from control.com
When methane gas is combusted, heat is released, making the reaction exothermic. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. Respiration is the chemical change. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. reactions that release energy:
Energy in Chemical Reactions Chemistry in Industrial Instrumentation
What Is A Reaction That Releases Energy when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. reactions that release energy: Respiration is the chemical change. when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. When methane gas is combusted, heat is released, making the reaction exothermic. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms.
From www.chemistrysteps.com
Energy and Organic Chemistry Reactions Chemistry Steps What Is A Reaction That Releases Energy for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. Respiration is the chemical. What Is A Reaction That Releases Energy.
From slideplayer.com
Chemical Reactions. ppt download What Is A Reaction That Releases Energy Respiration is the chemical change. reactions that release energy: When methane gas is combusted, heat is released, making the reaction exothermic. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. when the. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Chapter Chemical Reactions PowerPoint Presentation, free What Is A Reaction That Releases Energy In a combustion reaction, fuel is burned and reacts with oxygen to release energy. when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. When. What Is A Reaction That Releases Energy.
From slideplayer.com
2.4 Chemical Reactions and Enzymes ppt download What Is A Reaction That Releases Energy endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. When methane gas is combusted, heat is released, making the reaction exothermic. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. when the wood burns and forms carbon dioxide and water, it releases. What Is A Reaction That Releases Energy.
From www.numerade.com
SOLVEDA reaction that releases energy is called an reaction. What Is A Reaction That Releases Energy endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. reactions that release energy: for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In a. What Is A Reaction That Releases Energy.
From slideplayer.com
Chemical Reactions Chapter ppt download What Is A Reaction That Releases Energy In a combustion reaction, fuel is burned and reacts with oxygen to release energy. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. when the wood burns and forms carbon dioxide and water, it releases some. What Is A Reaction That Releases Energy.
From slideplayer.com
This presentation includes the following ppt download What Is A Reaction That Releases Energy endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. In a combustion reaction, fuel is burned. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Chemical Reactions & Enzymes PowerPoint Presentation, free What Is A Reaction That Releases Energy exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. reactions that release energy: In a combustion reaction, fuel is burned and reacts with oxygen to release energy. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Respiration is the chemical change. . What Is A Reaction That Releases Energy.
From www.chegg.com
Solved Chemical Reactions Occur When Molecules Or Atoms C... What Is A Reaction That Releases Energy When methane gas is combusted, heat is released, making the reaction exothermic. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. In a combustion reaction, fuel is burned and reacts. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Section 24 & 25 “Chemical Reactions & Enzymes ” PowerPoint What Is A Reaction That Releases Energy Respiration is the chemical change. when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In a combustion reaction, fuel is burned and reacts with oxygen to release. What Is A Reaction That Releases Energy.
From slideplayer.com
24 Chemical Reactions and Enzymes ppt download What Is A Reaction That Releases Energy exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. reactions that release energy: endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. when the wood burns and. What Is A Reaction That Releases Energy.
From spmchemistry.blog.onlinetuition.com.my
Energy Changes in Chemical Reaction SPM Chemistry What Is A Reaction That Releases Energy reactions that release energy: exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. When methane gas is combusted, heat is released, making the reaction exothermic. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. endothermic and exothermic reactions are chemical reactions. What Is A Reaction That Releases Energy.
From slideplayer.com
Chemical reactions and enzymes ppt download What Is A Reaction That Releases Energy Respiration is the chemical change. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. reactions that release energy: for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In a combustion reaction, fuel is burned and reacts with oxygen. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free What Is A Reaction That Releases Energy When methane gas is combusted, heat is released, making the reaction exothermic. reactions that release energy: Respiration is the chemical change. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. for example,. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Section 1 The Nature of Chemical Reactions PowerPoint What Is A Reaction That Releases Energy When methane gas is combusted, heat is released, making the reaction exothermic. Respiration is the chemical change. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. when the wood burns and forms carbon. What Is A Reaction That Releases Energy.
From slideplayer.com
CHEMICAL REACTIONS. ppt download What Is A Reaction That Releases Energy endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. reactions that release energy: When methane gas is combusted, heat is released, making the reaction exothermic. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. In a combustion reaction, fuel is burned and. What Is A Reaction That Releases Energy.
From control.com
Energy in Chemical Reactions Chemistry in Industrial Instrumentation What Is A Reaction That Releases Energy when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. When methane gas is. What Is A Reaction That Releases Energy.
From slideplayer.com
Section 2 Chemical Reactions ppt download What Is A Reaction That Releases Energy for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. When methane gas is combusted, heat is released, making the reaction exothermic. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. reactions that release energy: Respiration is the chemical change. exothermic and endothermic reactions can. What Is A Reaction That Releases Energy.
From slideplayer.com
Chapter 7 Chemical Reactions ppt download What Is A Reaction That Releases Energy for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. Respiration is the chemical change. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. When methane gas is combusted, heat is released, making the reaction exothermic. reactions that release energy: exothermic and endothermic reactions can. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Notes Energy & Chemical Change PowerPoint Presentation ID5643185 What Is A Reaction That Releases Energy for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. reactions that release energy: When methane gas is combusted, heat is released, making the reaction exothermic. exothermic and endothermic reactions can be thought of as having. What Is A Reaction That Releases Energy.
From slideplayer.com
Organic Chemistry, Chemical Reactions, and Enzymes ppt download What Is A Reaction That Releases Energy when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. reactions that release energy: In a combustion reaction, fuel is burned and reacts with oxygen to release energy. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. exothermic and. What Is A Reaction That Releases Energy.
From www.youtube.com
(5.10) Energy in Chemical Reactions YouTube What Is A Reaction That Releases Energy When methane gas is combusted, heat is released, making the reaction exothermic. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. when the wood burns and forms carbon dioxide and water, it releases. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Energy Changes in Reactions PowerPoint Presentation, free What Is A Reaction That Releases Energy reactions that release energy: Respiration is the chemical change. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. When methane gas is combusted, heat is released, making the reaction exothermic. when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some.. What Is A Reaction That Releases Energy.
From slideplayer.com
Chapter 7 Chemical Reactions ppt download What Is A Reaction That Releases Energy when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. exothermic and endothermic. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Chemical Reactions and Enzymes PowerPoint Presentation, free What Is A Reaction That Releases Energy when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. exothermic and endothermic reactions can be thought of as having energy as either a product of the. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID What Is A Reaction That Releases Energy endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. Respiration is the chemical change. when the wood burns and forms carbon dioxide. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT KEY CONCEPT Life depends on chemical reactions. PowerPoint What Is A Reaction That Releases Energy for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. When methane gas is combusted, heat is released, making the reaction exothermic. reactions that release energy: exothermic and endothermic reactions can be thought of as having. What Is A Reaction That Releases Energy.
From bio.libretexts.org
3.9 Energy in Chemical Reactions Biology LibreTexts What Is A Reaction That Releases Energy In a combustion reaction, fuel is burned and reacts with oxygen to release energy. Respiration is the chemical change. when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. When methane gas is combusted, heat is released, making the reaction exothermic. endothermic and exothermic reactions. What Is A Reaction That Releases Energy.
From slideplayer.com
Chemical Reactions. ppt download What Is A Reaction That Releases Energy endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Respiration is the chemical change. when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. for example,. What Is A Reaction That Releases Energy.
From slideplayer.com
24 Chemical Reactions and Enzymes ppt download What Is A Reaction That Releases Energy when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. Respiration is the chemical change. reactions that release energy: exothermic and endothermic reactions can be thought. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Ch. 16 Chemical Reactions PowerPoint Presentation, free What Is A Reaction That Releases Energy endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. reactions that release energy: When methane. What Is A Reaction That Releases Energy.
From slideplayer.com
Chemical Reactions. ppt download What Is A Reaction That Releases Energy when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. . What Is A Reaction That Releases Energy.
From slideplayer.com
Chemical Reactions and Enzymes ppt download What Is A Reaction That Releases Energy Respiration is the chemical change. reactions that release energy: when the wood burns and forms carbon dioxide and water, it releases some energy in the form of heat and light, while some. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In a combustion reaction, fuel is burned and. What Is A Reaction That Releases Energy.
From www.slideserve.com
PPT Energy, Chemical Reactions, and Enzymes PowerPoint Presentation What Is A Reaction That Releases Energy exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a. When methane gas is combusted, heat is released, making the reaction exothermic. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. for example, combustion reactions (redox reactions involving oxygen) are a major source. What Is A Reaction That Releases Energy.
From slideplayer.com
Chemical Reactions and Enzymes ppt download What Is A Reaction That Releases Energy When methane gas is combusted, heat is released, making the reaction exothermic. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. for example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. when the wood burns and forms carbon dioxide and water, it releases some energy in. What Is A Reaction That Releases Energy.