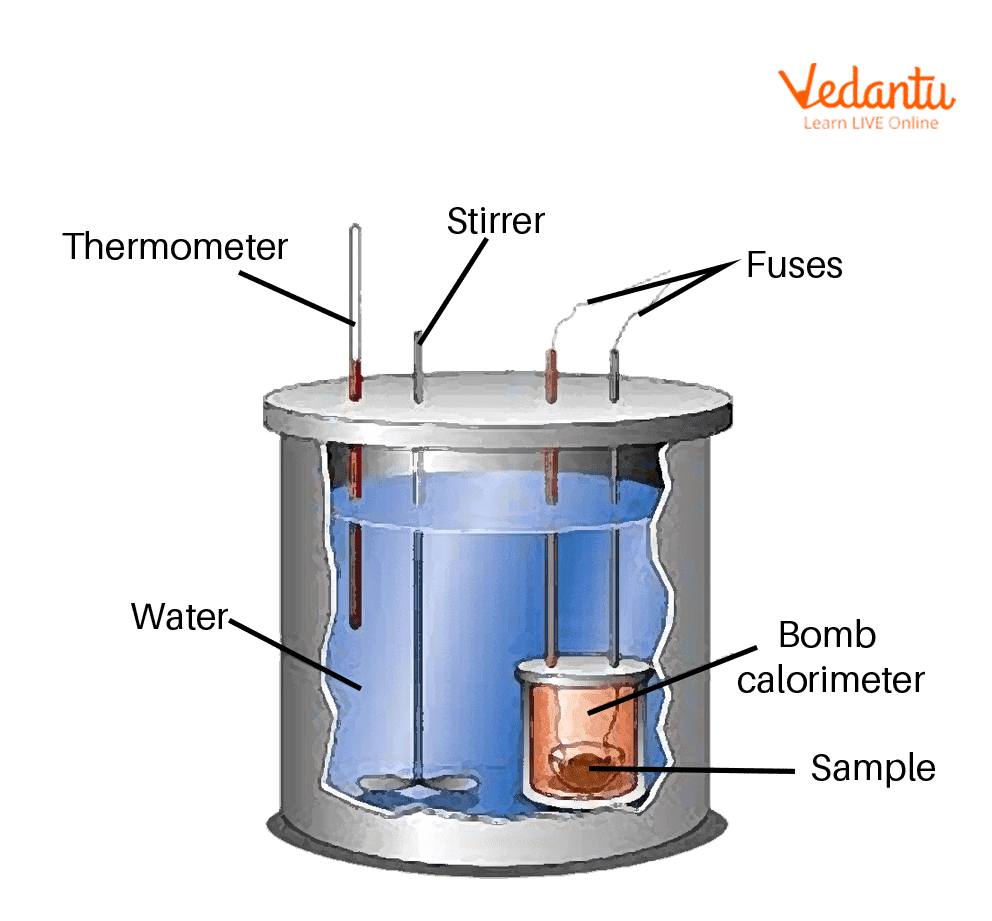
Bomb Calorimetry A Level . A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. A polystyrene cup can act as a. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. The burning sample heats a known. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. Determining the enthalpy of combustion of an alcohol. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. A tutorial guide on how to calculate the heat of combustion. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. This process involves burning a sample of a. The consequence of the calculation is called the amount of combustion, calorification, or btu. This lab demonstrates one of the most common. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can.
from www.vedantu.com
Determining the enthalpy of combustion of an alcohol. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. The burning sample heats a known. A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. A polystyrene cup can act as a. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. This lab demonstrates one of the most common.
Bomb Calorimeter Learn Important Terms and Concepts
Bomb Calorimetry A Level Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. This process involves burning a sample of a. Determining the enthalpy of combustion of an alcohol. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. The consequence of the calculation is called the amount of combustion, calorification, or btu. A tutorial guide on how to calculate the heat of combustion. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The burning sample heats a known. This lab demonstrates one of the most common. A polystyrene cup can act as a. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimetry A Level A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. A simple calorimeter can be made from a polystyrene drinking cup, a. Bomb Calorimetry A Level.
From drivenheisenberg.blogspot.com
Which Diagram Is A Bomb Calorimeter Drivenheisenberg Bomb Calorimetry A Level Determining the enthalpy of combustion of an alcohol. A tutorial guide on how to calculate the heat of combustion. The burning sample heats a known. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. Use our revision notes on calorimetry. Bomb Calorimetry A Level.
From saylordotorg.github.io
Calorimetry Bomb Calorimetry A Level Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. A tutorial guide on how to calculate the heat of combustion. The consequence of the calculation is called the amount of combustion, calorification, or btu. A polystyrene cup. Bomb Calorimetry A Level.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimetry A Level A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. Determining the enthalpy of combustion of an alcohol. The consequence of the calculation is. Bomb Calorimetry A Level.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Bomb Calorimetry A Level This process involves burning a sample of a. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. This lab demonstrates one of the most common. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). Bomb Calorimetry A Level.
From dxoazusse.blob.core.windows.net
Food Calorimetry Equation at Jesse Freeman blog Bomb Calorimetry A Level This process involves burning a sample of a. This lab demonstrates one of the most common. A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. A polystyrene cup can act as a. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and.. Bomb Calorimetry A Level.
From www.chegg.com
Solved Use the Bomb Calorimetry Interactive to answer the Bomb Calorimetry A Level This lab demonstrates one of the most common. Determining the enthalpy of combustion of an alcohol. This process involves burning a sample of a. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. A device used for experimental determination of. Bomb Calorimetry A Level.
From byjus.com
What is bomb calorimeter? Bomb Calorimetry A Level This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A tutorial guide on how to calculate the heat of combustion. Determining the enthalpy. Bomb Calorimetry A Level.
From www.savemyexams.co.uk
Biomass (5.3.3) AQA A Level Biology Revision Notes 2017 Save My Exams Bomb Calorimetry A Level This process involves burning a sample of a. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. A tutorial guide on how to. Bomb Calorimetry A Level.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Bomb Calorimetry A Level A polystyrene cup can act as a. This process involves burning a sample of a. The burning sample heats a known. The consequence of the calculation is called the amount of combustion, calorification, or btu. This lab demonstrates one of the most common. Determining the enthalpy of combustion of an alcohol. A simple calorimeter can be made from a polystyrene. Bomb Calorimetry A Level.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Bomb Calorimetry A Level A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. The burning sample heats a known. A polystyrene cup can act as a. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by. Bomb Calorimetry A Level.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog Bomb Calorimetry A Level This lab demonstrates one of the most common. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and.. Bomb Calorimetry A Level.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Bomb Calorimetry A Level A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Determining the enthalpy of combustion of an alcohol. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. A polystyrene cup can act as a. This. Bomb Calorimetry A Level.
From www.youtube.com
Bomb Calorimeter vs Coffee Cup Calorimeter Problem Constant Pressure Bomb Calorimetry A Level A tutorial guide on how to calculate the heat of combustion. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. A polystyrene cup can act as a. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. The burning sample heats a known. The. Bomb Calorimetry A Level.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Bomb Calorimetry A Level A tutorial guide on how to calculate the heat of combustion. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. A polystyrene cup can act as a. Determining the enthalpy of combustion of an alcohol. This process involves burning a sample of a. Bomb calorimetry uses a machine called a bomb calorimeter. Bomb Calorimetry A Level.
From worksheetmediadwaum.z14.web.core.windows.net
Calorimetry A Level Chemistry Questions Bomb Calorimetry A Level The consequence of the calculation is called the amount of combustion, calorification, or btu. The burning sample heats a known. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. Determining the enthalpy of combustion of an alcohol. Bomb calorimetry. Bomb Calorimetry A Level.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Bomb Calorimetry A Level This lab demonstrates one of the most common. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. A polystyrene cup can act as a. This involves burning the sample of dry biomass in a piece of equipment known as. Bomb Calorimetry A Level.
From exoefpebz.blob.core.windows.net
Bomb Calorimeter A Level Chemistry at Joseph Kiger blog Bomb Calorimetry A Level This process involves burning a sample of a. A polystyrene cup can act as a. The consequence of the calculation is called the amount of combustion, calorification, or btu. A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. This involves burning the sample of dry biomass in a piece of. Bomb Calorimetry A Level.
From www.studocu.com
Exp1 Bomb+Calorimetry A B Experiment 1 Determination of enthalpy of Bomb Calorimetry A Level The burning sample heats a known. The consequence of the calculation is called the amount of combustion, calorification, or btu. A polystyrene cup can act as a. This lab demonstrates one of the most common. Determining the enthalpy of combustion of an alcohol. A device used for experimental determination of heat change in the system is called a calorimeter (bomb. Bomb Calorimetry A Level.
From people.chem.umass.edu
to Adobe GoLive 6 Bomb Calorimetry A Level A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. This process involves burning a sample of a. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. This involves burning the sample of dry biomass in a piece of equipment known as. Bomb Calorimetry A Level.
From wiringdiagram99.blogspot.com
Which Diagram Is A Bomb Calorimeter Wiring Diagram Database Bomb Calorimetry A Level Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. A polystyrene cup can act as a. Determining the enthalpy of combustion of an alcohol. This. Bomb Calorimetry A Level.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimetry A Level A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can. A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed. Bomb Calorimetry A Level.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimetry A Level A polystyrene cup can act as a. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is used to measure, under controlled conditions,. Bomb Calorimetry A Level.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimetry A Level A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can. The burning sample heats a known. The consequence of the calculation is called the amount of combustion, calorification, or btu. A polystyrene cup can. Bomb Calorimetry A Level.
From courses.lumenlearning.com
5.2 Calorimetry Chemistry Bomb Calorimetry A Level The burning sample heats a known. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can. This process involves burning a sample of a. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter.. Bomb Calorimetry A Level.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimetry A Level The burning sample heats a known. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A tutorial guide on how to calculate the heat of combustion. A device used for experimental determination of heat change in the system is called. Bomb Calorimetry A Level.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Bomb Calorimetry A Level A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. This lab demonstrates one of the most common. This process involves burning a sample of a. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. This involves burning the sample of dry. Bomb Calorimetry A Level.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Bomb Calorimetry A Level A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. This process involves burning a sample of a. This lab demonstrates one of the most common. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. The consequence of the calculation is called the amount of. Bomb Calorimetry A Level.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 Bomb Calorimetry A Level This lab demonstrates one of the most common. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can. Determining the. Bomb Calorimetry A Level.
From www.linstitute.net
AQA A Level Biology复习笔记5.3.3 Biomass翰林国际教育 Bomb Calorimetry A Level A polystyrene cup can act as a. This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. The burning sample heats a known. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under. Bomb Calorimetry A Level.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimetry A Level This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. This lab demonstrates one of the most common. A polystyrene cup can act as a. Bomb calorimetry uses a machine called a bomb calorimeter. Bomb Calorimetry A Level.
From users.highland.edu
Calorimetry Bomb Calorimetry A Level A tutorial guide on how to calculate the heat of combustion. A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. This involves burning the sample of dry biomass in a piece of equipment known as. Bomb Calorimetry A Level.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Bomb Calorimetry A Level Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of combustion. The burning sample heats a known. Determining the enthalpy of combustion of benzoic acid with a bomb calorimeter. This process involves burning a sample of a. Determining the enthalpy of combustion of an alcohol. Use our revision notes on calorimetry for a level chemistry to. Bomb Calorimetry A Level.
From ivypanda.com
Bomb Calorimetry Theory and Experiment 1595 Words Report Example Bomb Calorimetry A Level A device used for experimental determination of heat change in the system is called a calorimeter (bomb calorimeter), a. A polystyrene cup can act as a. The burning sample heats a known. The consequence of the calculation is called the amount of combustion, calorification, or btu. Bomb calorimetry uses a machine called a bomb calorimeter to measure enthalpy changes of. Bomb Calorimetry A Level.
From www.chegg.com
Solved The Model Bomb Calorimetry Motorized stirrer Bomb Calorimetry A Level This involves burning the sample of dry biomass in a piece of equipment known as a calorimeter. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Determining the enthalpy of combustion of an alcohol. Bomb calorimetry uses a machine called. Bomb Calorimetry A Level.