Chlorine Neutral Charge . There are four ways to find the charge of an element: A chlorine atom starts with 17 electrons and 17 protons and is neutral. This table shows the most common charges for atoms of the chemical elements. After gaining an electron to become an ion, it now. 93 rows updated on may 07, 2024. The usual charge of an element is common to its group. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Therefore, the number of electrons in neutral atom of chlorine is 17. You can use this table to predict whether an atom can bond with another. A neutral chlorine atom has seven electrons in its outermost shell. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. In the united states, it is almost. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. Only one more electron is needed to achieve an octet in.
from oerpub.github.io
A neutral chlorine atom has seven electrons in its outermost shell. 93 rows updated on may 07, 2024. After gaining an electron to become an ion, it now. Only one more electron is needed to achieve an octet in. Therefore, the number of electrons in neutral atom of chlorine is 17. The usual charge of an element is common to its group. There are four ways to find the charge of an element: A chlorine atom starts with 17 electrons and 17 protons and is neutral. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. You can use this table to predict whether an atom can bond with another.
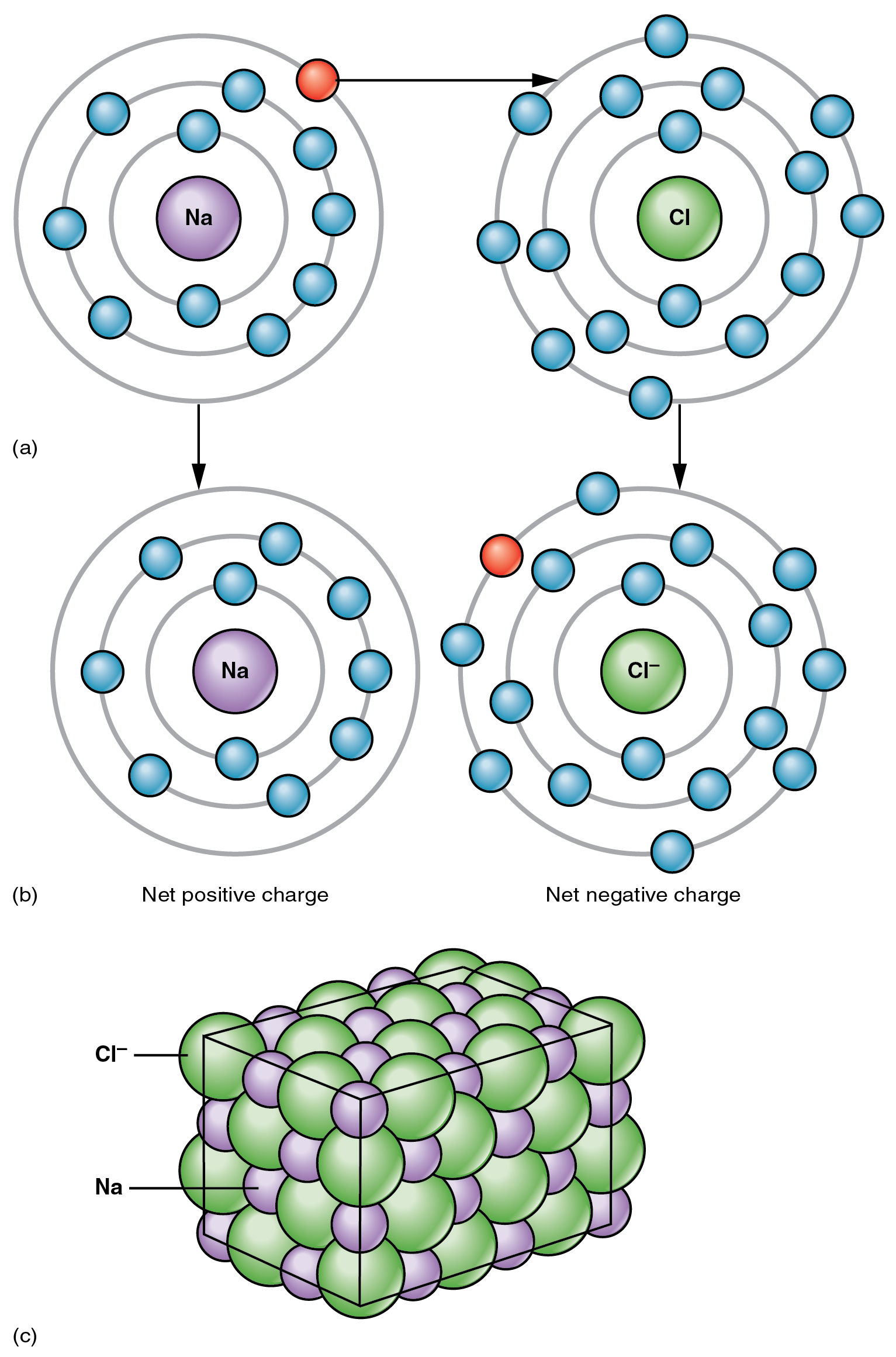
The top panel of this figure shows the orbit model of a sodium atom and
Chlorine Neutral Charge A chlorine atom starts with 17 electrons and 17 protons and is neutral. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. You can use this table to predict whether an atom can bond with another. A neutral chlorine atom has seven electrons in its outermost shell. After gaining an electron to become an ion, it now. This table shows the most common charges for atoms of the chemical elements. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. 93 rows updated on may 07, 2024. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. A chlorine atom starts with 17 electrons and 17 protons and is neutral. In the united states, it is almost. Therefore, the number of electrons in neutral atom of chlorine is 17. Only one more electron is needed to achieve an octet in. The usual charge of an element is common to its group. There are four ways to find the charge of an element:
From www.nuclear-power.com
What is Chlorine Properties of Chlorine Element Symbol Cl nuclear Chlorine Neutral Charge Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. In the united states, it is almost. The usual charge of an element is common to its group. There are four ways to find the charge of an element: A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Therefore, the number of electrons. Chlorine Neutral Charge.
From www.numerade.com
SOLVED The sodium had a neutral charge when it had one electron on its Chlorine Neutral Charge A neutral chlorine atom has seven electrons in its outermost shell. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. After gaining an electron to become an ion, it now. There are four ways to find the charge of an element: Only one more electron is needed to achieve an octet in. In the united states, it. Chlorine Neutral Charge.
From www.chansonwaterusa.com
The Hazards of Breathing Chlorine Vapor Chanson Naturals Chlorine Neutral Charge A neutral chlorine atom, for example, contains 17 protons and 17 electrons. You can use this table to predict whether an atom can bond with another. A neutral chlorine atom has seven electrons in its outermost shell. This table shows the most common charges for atoms of the chemical elements. Only one more electron is needed to achieve an octet. Chlorine Neutral Charge.
From slideplayer.com
What are ionic compounds and how do they form? ppt download Chlorine Neutral Charge Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. In the united states, it is almost. Therefore, the number of electrons in neutral atom of chlorine is 17. Only one more electron is needed to achieve an octet in. A neutral chlorine atom has seven electrons in its outermost shell. The usual charge of an. Chlorine Neutral Charge.
From www.chegg.com
Solved Chlorine forms an ion with a charge of? a. 7− b. 2+ Chlorine Neutral Charge A neutral chlorine atom, for example, contains 17 protons and 17 electrons. In the united states, it is almost. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. A neutral chlorine atom has seven electrons in its outermost shell. You can use this table to predict whether an atom can bond with another. Only one. Chlorine Neutral Charge.
From www.numerade.com
SOLVED The electron dot diagram for a neutral atom of chlorine (atomic Chlorine Neutral Charge After gaining an electron to become an ion, it now. In the united states, it is almost. This table shows the most common charges for atoms of the chemical elements. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. You can. Chlorine Neutral Charge.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Chlorine Neutral Charge This table shows the most common charges for atoms of the chemical elements. The usual charge of an element is common to its group. There are four ways to find the charge of an element: Therefore, the number of electrons in neutral atom of chlorine is 17. In the united states, it is almost. Each electron is influenced by the. Chlorine Neutral Charge.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Neutral Charge 93 rows updated on may 07, 2024. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. Only one more electron is needed to achieve an octet in. After gaining an electron to become an ion, it now. Therefore, the number of electrons in neutral atom of chlorine is 17. There are four ways to find. Chlorine Neutral Charge.
From www.numerade.com
SOLVED What happens when a chloride ion forms? Group of answer choices Chlorine Neutral Charge A neutral chlorine atom has seven electrons in its outermost shell. You can use this table to predict whether an atom can bond with another. After gaining an electron to become an ion, it now. There are four ways to find the charge of an element: Therefore, the number of electrons in neutral atom of chlorine is 17. The usual. Chlorine Neutral Charge.
From cartoondealer.com
Anions And Cations For Example Sodium And Chlorine Atoms. Cartoon Chlorine Neutral Charge Therefore, the number of electrons in neutral atom of chlorine is 17. A neutral chlorine atom has seven electrons in its outermost shell. After gaining an electron to become an ion, it now. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. You can use this table to predict whether an atom can bond with. Chlorine Neutral Charge.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains Chlorine Neutral Charge In the united states, it is almost. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. 93 rows updated on may 07, 2024. After gaining an electron to become an ion, it now. Only one more electron is needed to achieve an octet in. Therefore, the number of electrons in neutral atom of chlorine is. Chlorine Neutral Charge.
From gardenandplate.com
Molecules Chlorine Neutral Charge Therefore, the number of electrons in neutral atom of chlorine is 17. You can use this table to predict whether an atom can bond with another. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. A chlorine atom starts with 17 electrons and 17 protons and is neutral. Chlorine is used in the. Chlorine Neutral Charge.
From slideplayer.com
Ionic Bonding. ppt download Chlorine Neutral Charge Each electron is influenced by the electric fields produced by the positive nuclear charge and the. There are four ways to find the charge of an element: Only one more electron is needed to achieve an octet in. A chlorine atom starts with 17 electrons and 17 protons and is neutral. Therefore, the number of electrons in neutral atom of. Chlorine Neutral Charge.
From quizizz.com
Properties of Ionic Compounds Chemistry Quiz Quizizz Chlorine Neutral Charge This table shows the most common charges for atoms of the chemical elements. The usual charge of an element is common to its group. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. In the united states, it is almost. A neutral chlorine atom has seven electrons in its outermost shell. You can use this table to. Chlorine Neutral Charge.
From sciencenotes.org
Chlorine Facts Chlorine Neutral Charge In the united states, it is almost. A neutral chlorine atom has seven electrons in its outermost shell. A chlorine atom starts with 17 electrons and 17 protons and is neutral. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. The usual charge. Chlorine Neutral Charge.
From mavink.com
Aufbau Diagram For Chlorine Chlorine Neutral Charge There are four ways to find the charge of an element: A chlorine atom starts with 17 electrons and 17 protons and is neutral. The usual charge of an element is common to its group. Only one more electron is needed to achieve an octet in. 93 rows updated on may 07, 2024. Chlorine is used in the disinfection (removal. Chlorine Neutral Charge.
From socratic.org
Question 587a9 Socratic Chlorine Neutral Charge A chlorine atom starts with 17 electrons and 17 protons and is neutral. After gaining an electron to become an ion, it now. Therefore, the number of electrons in neutral atom of chlorine is 17. Only one more electron is needed to achieve an octet in. You can use this table to predict whether an atom can bond with another.. Chlorine Neutral Charge.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Chlorine Neutral Charge Only one more electron is needed to achieve an octet in. There are four ways to find the charge of an element: A neutral chlorine atom has seven electrons in its outermost shell. In the united states, it is almost. This table shows the most common charges for atoms of the chemical elements. The usual charge of an element is. Chlorine Neutral Charge.
From slideplayer.com
Chemistry Topic 6 Chemical Compounds. ppt download Chlorine Neutral Charge Therefore, the number of electrons in neutral atom of chlorine is 17. After gaining an electron to become an ion, it now. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. This table shows the most common charges for atoms of the chemical elements. Chlorine is used in the disinfection (removal of harmful. Chlorine Neutral Charge.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Neutral Charge The usual charge of an element is common to its group. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. A neutral chlorine atom has seven electrons in its outermost shell. This table shows the most common charges for atoms of the chemical elements. In the united states, it is almost. Therefore, the. Chlorine Neutral Charge.
From courses.lumenlearning.com
6.1 Lewis Electron Dot Symbols Introductory Chemistry Chlorine Neutral Charge You can use this table to predict whether an atom can bond with another. This table shows the most common charges for atoms of the chemical elements. A chlorine atom starts with 17 electrons and 17 protons and is neutral. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. The usual charge of an element. Chlorine Neutral Charge.
From saylordotorg.github.io
Ions Chlorine Neutral Charge After gaining an electron to become an ion, it now. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. There are four ways to find the charge of an element: A neutral chlorine atom, for example, contains 17 protons and 17 electrons. Therefore, the number of electrons in neutral atom of chlorine is 17. This. Chlorine Neutral Charge.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Chlorine Have? Chlorine Neutral Charge You can use this table to predict whether an atom can bond with another. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. Therefore, the number of electrons in neutral atom of chlorine is 17. Only one more electron is needed to achieve an octet in. 93 rows updated on may 07, 2024. A chlorine. Chlorine Neutral Charge.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Neutral Charge Each electron is influenced by the electric fields produced by the positive nuclear charge and the. A neutral chlorine atom has seven electrons in its outermost shell. Therefore, the number of electrons in neutral atom of chlorine is 17. Only one more electron is needed to achieve an octet in. Chlorine is used in the disinfection (removal of harmful microorganisms). Chlorine Neutral Charge.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Neutral Charge Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. You can use this table to predict whether an atom can bond with another. Only one more electron is needed to achieve an octet in. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. Therefore, the number of electrons. Chlorine Neutral Charge.
From www.nuclear-power.com
Fluorine Electron Affinity Electronegativity Ionization Energy of Chlorine Neutral Charge A chlorine atom starts with 17 electrons and 17 protons and is neutral. The usual charge of an element is common to its group. You can use this table to predict whether an atom can bond with another. After gaining an electron to become an ion, it now. A neutral chlorine atom has seven electrons in its outermost shell. Only. Chlorine Neutral Charge.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Neutral Charge 93 rows updated on may 07, 2024. Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. Therefore, the number of electrons in neutral atom of chlorine is 17. A neutral chlorine atom, for example, contains 17 protons and 17. Chlorine Neutral Charge.
From mavink.com
Aufbau Diagram For Chlorine Chlorine Neutral Charge There are four ways to find the charge of an element: Only one more electron is needed to achieve an octet in. 93 rows updated on may 07, 2024. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. After gaining an electron to become an ion, it now. Therefore, the number of electrons in neutral atom of. Chlorine Neutral Charge.
From www.numerade.com
SOLVED The electron dot diagram for a neutral atom of chlorine (atomic Chlorine Neutral Charge Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. A neutral chlorine atom has seven electrons in its outermost shell. After gaining an electron to become an ion, it now. There are four ways to find the charge of an element: You can use this table to predict whether an atom can bond with another.. Chlorine Neutral Charge.
From basichemistry.blogspot.com
Basic Chemistry October 2012 Chlorine Neutral Charge Each electron is influenced by the electric fields produced by the positive nuclear charge and the. You can use this table to predict whether an atom can bond with another. After gaining an electron to become an ion, it now. A chlorine atom starts with 17 electrons and 17 protons and is neutral. This table shows the most common charges. Chlorine Neutral Charge.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Neutral Charge Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. 93 rows updated on may 07, 2024. This table shows the most common charges for atoms of the chemical elements. After gaining an electron to become an ion, it now. Therefore, the number of electrons in neutral atom of chlorine is 17. You can use this. Chlorine Neutral Charge.
From loenqakuj.blob.core.windows.net
Chlorine Number For Atomic Mass at Pamela Lansing blog Chlorine Neutral Charge Only one more electron is needed to achieve an octet in. This table shows the most common charges for atoms of the chemical elements. A neutral chlorine atom has seven electrons in its outermost shell. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. There are four ways to find the charge of an element: A chlorine. Chlorine Neutral Charge.
From wisc.pb.unizin.org
Resonance Structures and Formal Charge (M8Q3) UWMadison Chemistry Chlorine Neutral Charge Each electron is influenced by the electric fields produced by the positive nuclear charge and the. This table shows the most common charges for atoms of the chemical elements. Only one more electron is needed to achieve an octet in. 93 rows updated on may 07, 2024. After gaining an electron to become an ion, it now. There are four. Chlorine Neutral Charge.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Chlorine Neutral Charge Chlorine is used in the disinfection (removal of harmful microorganisms) of water and wastewater. The usual charge of an element is common to its group. There are four ways to find the charge of an element: You can use this table to predict whether an atom can bond with another. A neutral chlorine atom, for example, contains 17 protons and. Chlorine Neutral Charge.
From slideplayer.com
II. Ionic Bonds Part ppt download Chlorine Neutral Charge Therefore, the number of electrons in neutral atom of chlorine is 17. You can use this table to predict whether an atom can bond with another. A chlorine atom starts with 17 electrons and 17 protons and is neutral. Each electron is influenced by the electric fields produced by the positive nuclear charge and the. Chlorine is used in the. Chlorine Neutral Charge.