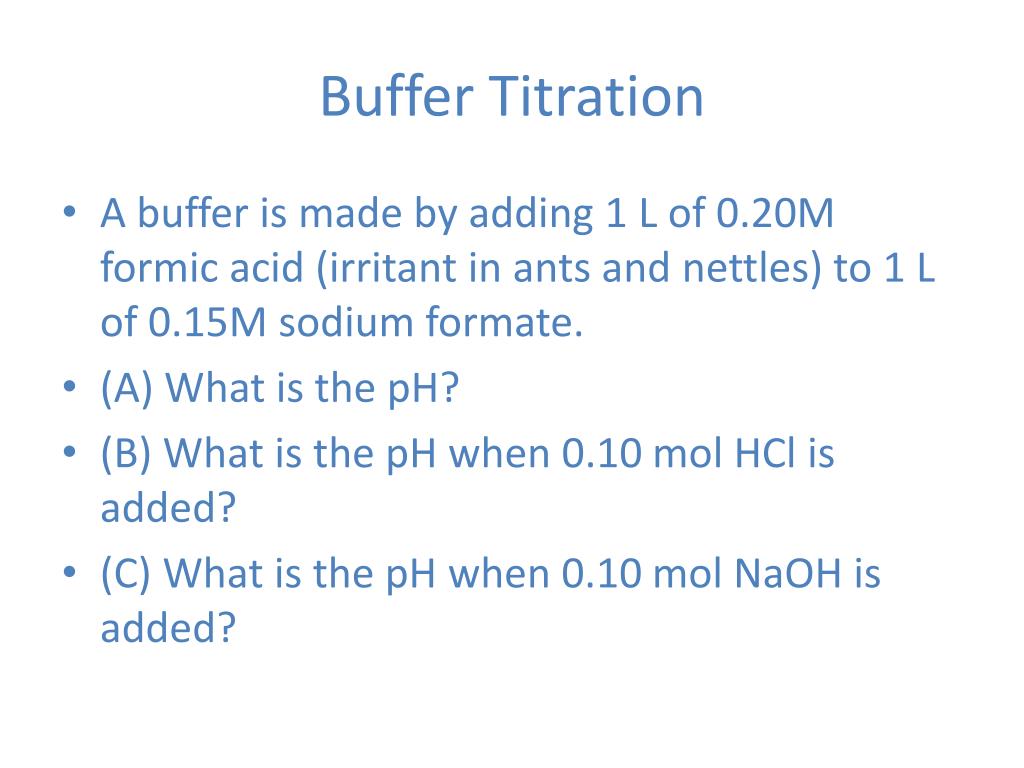
Buffer Titration Problems . Use the titrations on the web site (move the slider bar to. If ha is titrated with naoh, as soon as. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Weak acids/bases titrated with strong acids/bases twelve examples. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. All examples and problems, no solutions. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution).
from www.slideserve.com
In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. If ha is titrated with naoh, as soon as. Weak acids/bases titrated with strong acids/bases twelve examples. Use the titrations on the web site (move the slider bar to. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. All examples and problems, no solutions.
PPT Unit 122 PowerPoint Presentation, free download ID1994619
Buffer Titration Problems Weak acids/bases titrated with strong acids/bases twelve examples. If ha is titrated with naoh, as soon as. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). All examples and problems, no solutions. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Weak acids/bases titrated with strong acids/bases twelve examples. Use the titrations on the web site (move the slider bar to.
From www.slideserve.com
PPT Buffers, Titrations, and Aqueous Equilibria PowerPoint Buffer Titration Problems Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The shape of a titration curve, a plot of ph versus the amount. Buffer Titration Problems.
From www.studocu.com
Test3 ch17b BufferTitrationEquilibrium Practice Problems General Buffer Titration Problems All examples and problems, no solutions. Use the titrations on the web site (move the slider bar to. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Any titration involving a weak a/b, from the first drop to the last, before. Buffer Titration Problems.
From www.chegg.com
Buffers, Titrations, and Solubility Solved Problems Buffer Titration Problems Weak acids/bases titrated with strong acids/bases twelve examples. If ha is titrated with naoh, as soon as. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. All examples and problems, no solutions. The shape of a titration curve, a plot of ph versus the amount of acid or base added,. Buffer Titration Problems.
From www.studypool.com
SOLUTION Titrations and buffers problems Studypool Buffer Titration Problems Use the titrations on the web site (move the slider bar to. Weak acids/bases titrated with strong acids/bases twelve examples. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. In this section, we will see how to perform calculations to predict the ph at any point in a titration of. Buffer Titration Problems.
From www.showme.com
Titration / Buffer Practice Science, Chemicalreactions, Titration Buffer Titration Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Weak acids/bases titrated with strong acids/bases twelve examples. The shape of a. Buffer Titration Problems.
From klajqxgeq.blob.core.windows.net
Titration Question Examples at Larry Magno blog Buffer Titration Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Weak acids/bases titrated with strong acids/bases twelve. Buffer Titration Problems.
From www.chegg.com
Solved Buffer problems and preliminary titration stuff... Buffer Titration Problems The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Weak acids/bases titrated with strong acids/bases twelve examples. If ha is titrated with naoh, as soon as. All examples and problems, no solutions. In this section, we will see how to perform calculations to predict. Buffer Titration Problems.
From www.coursehero.com
[Solved] Buffers and Titrations 1. What is a Buffer System, how do we Buffer Titration Problems The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base,. Buffer Titration Problems.
From www.slideserve.com
PPT Buffer or Common Ion problems. PowerPoint Presentation, free Buffer Titration Problems Weak acids/bases titrated with strong acids/bases twelve examples. All examples and problems, no solutions. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). If ha is titrated with naoh, as soon as. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a. Buffer Titration Problems.
From www.coursehero.com
[Solved] Buffers and Titrations 1. What is a Buffer System, how do we Buffer Titration Problems The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Use the titrations on the web site (move the slider bar to. All examples and problems,. Buffer Titration Problems.
From www.studocu.com
Titration and buffers Mixing acids and bases (titrations and other Buffer Titration Problems All examples and problems, no solutions. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Explain how a buffer solution manages to. Buffer Titration Problems.
From www.chegg.com
Buffers, Titrations, and Solubility Solved Problems Buffer Titration Problems All examples and problems, no solutions. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Use the titrations on the web site. Buffer Titration Problems.
From pubs.acs.org
Incorporating GuidedInquiry Experimental Design into a Traditional Buffer Titration Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Use the titrations on the web site (move the slider bar to. In this section,. Buffer Titration Problems.
From www.slideserve.com
PPT Buffer or Common Ion problems. PowerPoint Presentation, free Buffer Titration Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). If ha is titrated with naoh, as soon as. Any titration involving. Buffer Titration Problems.
From www.slideserve.com
PPT Buffer or Common Ion problems. PowerPoint Presentation, free Buffer Titration Problems Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Weak acids/bases titrated with strong acids/bases twelve examples. Any titration involving a. Buffer Titration Problems.
From www.slideserve.com
PPT Acid Base Equilibria , Buffers, Titration PowerPoint Presentation Buffer Titration Problems The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. If ha is titrated with naoh, as soon as. Use the titrations on the web site. Buffer Titration Problems.
From www.chegg.com
Solved Buffers, Titrations, and Solubility Solved Problems Buffer Titration Problems If ha is titrated with naoh, as soon as. Weak acids/bases titrated with strong acids/bases twelve examples. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). All examples and problems,. Buffer Titration Problems.
From saylordotorg.github.io
Buffers Buffer Titration Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Weak acids/bases titrated with strong acids/bases twelve examples. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Buffer Titration Problems.
From www.chegg.com
Solved Buffers, Titrations, and Solubility Solved Problems Buffer Titration Problems If ha is titrated with naoh, as soon as. All examples and problems, no solutions. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or. Buffer Titration Problems.
From mavink.com
Buffer Region Titration Curve Buffer Titration Problems If ha is titrated with naoh, as soon as. Weak acids/bases titrated with strong acids/bases twelve examples. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. All examples and problems, no solutions. The shape of a titration curve, a plot of. Buffer Titration Problems.
From www.tes.com
BUFFERS, TITRATIONS POWER POINT pH Salts Grade 12 Chemistry Power Point Buffer Titration Problems If ha is titrated with naoh, as soon as. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). All examples and problems, no solutions. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. In this section, we will see. Buffer Titration Problems.
From slidetodoc.com
Applications of Aqueous Equilibria Buffers AcidBase Titrations and Buffer Titration Problems The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. If ha is titrated with naoh, as. Buffer Titration Problems.
From www.reddit.com
Struggling with buffers? Check out these simple tricks to tackling Buffer Titration Problems Weak acids/bases titrated with strong acids/bases twelve examples. If ha is titrated with naoh, as soon as. Use the titrations on the web site (move the slider bar to. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Any titration involving a weak a/b,. Buffer Titration Problems.
From www.studocu.com
217 buffer, titration problems CHEM 217 Studocu Buffer Titration Problems Use the titrations on the web site (move the slider bar to. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak. Buffer Titration Problems.
From studylib.net
Buffers, Titrations & Indicators Buffer Titration Problems All examples and problems, no solutions. If ha is titrated with naoh, as soon as. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution).. Buffer Titration Problems.
From www.studypool.com
SOLUTION Titrations and buffers problems Studypool Buffer Titration Problems Weak acids/bases titrated with strong acids/bases twelve examples. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. If ha is titrated with naoh, as soon as. Explain how a buffer solution manages to stabilize the ph against the addition of acid,. Buffer Titration Problems.
From www.slideserve.com
PPT Unit 122 PowerPoint Presentation, free download ID1994619 Buffer Titration Problems If ha is titrated with naoh, as soon as. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Weak acids/bases titrated with strong acids/bases twelve examples. Use the titrations on the web site (move the slider bar to. Any titration involving a weak a/b,. Buffer Titration Problems.
From www.studypool.com
SOLUTION Chemical engineering review ph of monoprotic acid ph of Buffer Titration Problems All examples and problems, no solutions. Use the titrations on the web site (move the slider bar to. If ha is titrated with naoh, as soon as. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Weak acids/bases titrated with strong acids/bases twelve examples. The shape of a titration. Buffer Titration Problems.
From www.youtube.com
GCII Buffers and Titrations practice problems. YouTube Buffer Titration Problems All examples and problems, no solutions. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Weak acids/bases titrated with strong acids/bases twelve examples. Use the titrations on the web site (move the slider bar to. Any titration involving a weak a/b, from the first drop to the last, before. Buffer Titration Problems.
From www.sliderbase.com
Buffers Presentation Chemistry Buffer Titration Problems Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. If ha is titrated with naoh, as soon as. All examples and problems, no solutions. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Use the titrations on the web. Buffer Titration Problems.
From www.chegg.com
Solved EXP. 5 BUFFERS, TITRATION CURVES, AND INDICATORS LAB Buffer Titration Problems Use the titrations on the web site (move the slider bar to. If ha is titrated with naoh, as soon as. Weak acids/bases titrated with strong acids/bases twelve examples. All examples and problems, no solutions. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In this section, we will. Buffer Titration Problems.
From www.slideserve.com
PPT Acid Base Equilibria , Buffers, Titration PowerPoint Presentation Buffer Titration Problems Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Use the titrations on the web site (move the slider bar to. All examples and problems, no solutions. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). If ha is. Buffer Titration Problems.
From www.slideserve.com
PPT Acid Base Equilibria , Buffers, Titration PowerPoint Presentation Buffer Titration Problems In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Use the titrations on the web site (move the slider bar to.. Buffer Titration Problems.
From www.slideserve.com
PPT Acid Base Equilibria , Buffers, Titration PowerPoint Presentation Buffer Titration Problems Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. If ha is titrated with naoh, as soon as. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Explain how a buffer. Buffer Titration Problems.
From www.studocu.com
Buffers Practice Buffers and Titrations Extra Practice Problems What Buffer Titration Problems The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring. Weak acids/bases titrated with strong acids/bases twelve examples. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Use the titrations on the web site. Buffer Titration Problems.