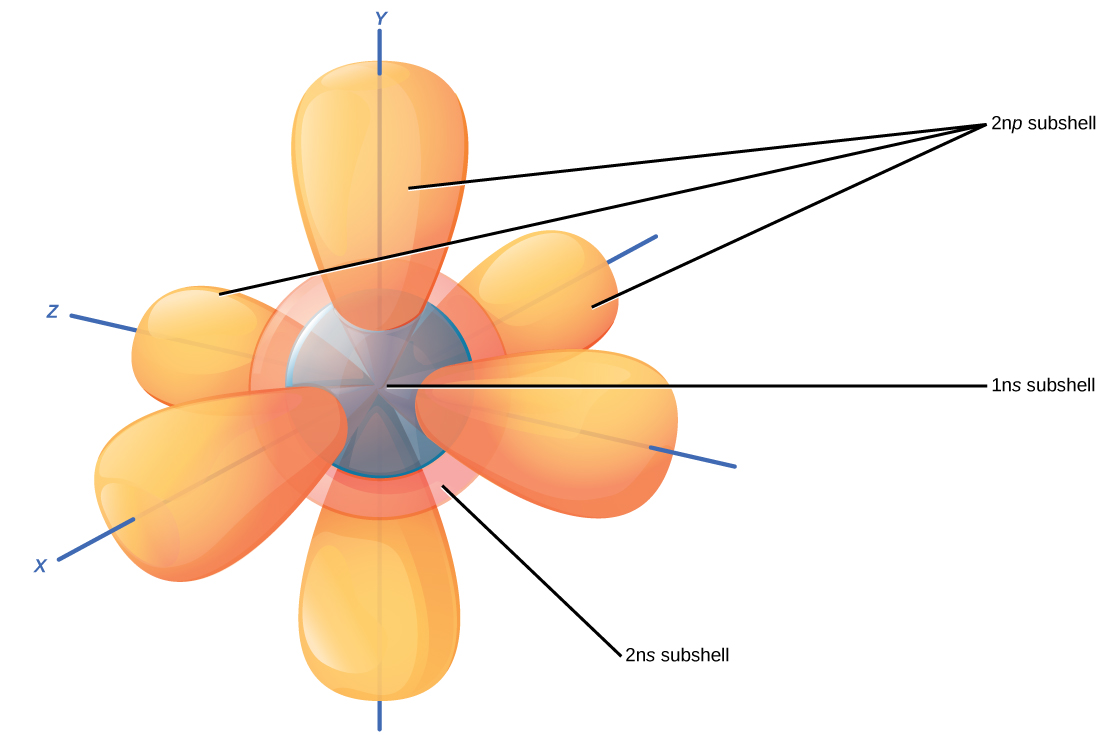
How Does Electron Promotion Work . The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence. Therefore, energy is actually released when carbon. Since electrons are indisinguishable and don't have specific. Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. It can be gained or lost through molecular collisions and heat transfer. A key point is that electrons do not actually reside in specific orbitals. In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that would be appropriate to promote an electron to a higher energy level. Vibrational energy, however, is not exchanged exclusively by means of photons. When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)).
from courses.lumenlearning.com
When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence. Since electrons are indisinguishable and don't have specific. It can be gained or lost through molecular collisions and heat transfer. In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that would be appropriate to promote an electron to a higher energy level. Therefore, energy is actually released when carbon. A key point is that electrons do not actually reside in specific orbitals. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. Vibrational energy, however, is not exchanged exclusively by means of photons. Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one.
Electrons Biology for Majors I
How Does Electron Promotion Work When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Vibrational energy, however, is not exchanged exclusively by means of photons. In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that would be appropriate to promote an electron to a higher energy level. A key point is that electrons do not actually reside in specific orbitals. When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). Since electrons are indisinguishable and don't have specific. It can be gained or lost through molecular collisions and heat transfer. Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. Therefore, energy is actually released when carbon.
From www.youtube.com
Splitting of Spectroscopic Term S,P,D&F(Electron promotion&Hole How Does Electron Promotion Work When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). A key point is that electrons do not actually reside in specific orbitals. Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence. The. How Does Electron Promotion Work.
From www.savemyexams.com
Colour in Transition Metal Complexes (HL) HL IB Chemistry Revision How Does Electron Promotion Work Therefore, energy is actually released when carbon. When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). A key point is that electrons do not actually reside in specific orbitals. It can be gained or lost through molecular collisions and heat transfer. Different wavelengths would be able. How Does Electron Promotion Work.
From www.numerade.com
SOLVED Question 14 (5 points) Which is the proper sequence of events How Does Electron Promotion Work Therefore, energy is actually released when carbon. Vibrational energy, however, is not exchanged exclusively by means of photons. Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a. How Does Electron Promotion Work.
From www.slideserve.com
PPT Early Chemistry PowerPoint Presentation ID544084 How Does Electron Promotion Work When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). A key point is that electrons do not actually reside in specific orbitals. Vibrational energy, however, is not exchanged exclusively by means of photons. Electron promotion is when an electron absorbs a photon to jump from a. How Does Electron Promotion Work.
From slideplayer.com
VSEPR Theory Valence Shell Electron Pair Repulsion theory ppt video How Does Electron Promotion Work Since electrons are indisinguishable and don't have specific. It can be gained or lost through molecular collisions and heat transfer. Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. In the case of visible or ultraviolet light, the energy of a photon is roughly in. How Does Electron Promotion Work.
From www.researchgate.net
Electron promotion and fragmentation scheme of region A in Fig. 11 [6 How Does Electron Promotion Work The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence.. How Does Electron Promotion Work.
From www.researchgate.net
(PDF) Electron promotion and electronic friction in atomic collision How Does Electron Promotion Work The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence.. How Does Electron Promotion Work.
From www.numerade.com
SOLVED please help asap and draw orbital diagram Look at the compound How Does Electron Promotion Work Vibrational energy, however, is not exchanged exclusively by means of photons. Therefore, energy is actually released when carbon. When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that. How Does Electron Promotion Work.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho How Does Electron Promotion Work Since electrons are indisinguishable and don't have specific. A key point is that electrons do not actually reside in specific orbitals. Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Electron promotion is when an electron absorbs a photon to jump from a low energy. How Does Electron Promotion Work.
From www.semanticscholar.org
Figure 3 from Promotion in solid phase reaction of Pt/SiOx bilayer film How Does Electron Promotion Work In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that would be appropriate to promote an electron to a higher energy level. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n −. How Does Electron Promotion Work.
From www.slideserve.com
PPT Chapter 3. MOLECULAR SHAPE AND STRUCTURE PowerPoint Presentation How Does Electron Promotion Work Vibrational energy, however, is not exchanged exclusively by means of photons. It can be gained or lost through molecular collisions and heat transfer. Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Therefore, energy is actually released when carbon. Electron promotion is when an electron. How Does Electron Promotion Work.
From www.science.org
Vibrational Promotion of Electron Transfer Science How Does Electron Promotion Work When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that would be appropriate to promote an electron to a higher energy level. Vibrational energy, however, is not exchanged. How Does Electron Promotion Work.
From www.youtube.com
Grade 11 Chemistry Electron Promotion & Hybridization YouTube How Does Electron Promotion Work It can be gained or lost through molecular collisions and heat transfer. Therefore, energy is actually released when carbon. Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Vibrational energy, however, is not exchanged exclusively by means of photons. Electron promotion is when an electron. How Does Electron Promotion Work.
From www.semanticscholar.org
Figure 1 from Promotion in solid phase reaction of Pt/SiOx bilayer film How Does Electron Promotion Work When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). It can be gained or lost through molecular collisions and heat transfer. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty. How Does Electron Promotion Work.
From courses.lumenlearning.com
Electrons Biology for Majors I How Does Electron Promotion Work Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence. The formation of hybrid atomic orbitals can be viewed as occurring via promotion. How Does Electron Promotion Work.
From studylib.net
Electron Arrangements How Does Electron Promotion Work It can be gained or lost through molecular collisions and heat transfer. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. Vibrational energy, however, is not exchanged exclusively by means of photons.. How Does Electron Promotion Work.
From www.numerade.com
SOLVED Question 14 (5 points) Which is the proper sequence of events How Does Electron Promotion Work Therefore, energy is actually released when carbon. It can be gained or lost through molecular collisions and heat transfer. Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence. When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational. How Does Electron Promotion Work.
From www.toppr.com
Electron promotion rule forbids the transition How Does Electron Promotion Work In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that would be appropriate to promote an electron to a higher energy level. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n −. How Does Electron Promotion Work.
From chem.libretexts.org
Chapter 6.2 Hybrid Orbitals Chemistry LibreTexts How Does Electron Promotion Work Since electrons are indisinguishable and don't have specific. Therefore, energy is actually released when carbon. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. In the case of visible or ultraviolet light,. How Does Electron Promotion Work.
From www.slideserve.com
PPT Light absorption PowerPoint Presentation ID2699464 How Does Electron Promotion Work Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Vibrational energy, however, is not exchanged exclusively by means of photons. Since electrons are indisinguishable and don't have specific. A key point is that electrons do not actually reside in specific orbitals. In the case of. How Does Electron Promotion Work.
From www.savemyexams.com
Coloured Complexes CIE A Level Chemistry Revision Notes 2022 How Does Electron Promotion Work When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). Since electrons are indisinguishable and don't have specific. In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that would be appropriate to promote an electron to a higher. How Does Electron Promotion Work.
From slideplayer.com
13/11/ ppt download How Does Electron Promotion Work Vibrational energy, however, is not exchanged exclusively by means of photons. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. Therefore, energy is actually released when carbon. When an electron is promoted. How Does Electron Promotion Work.
From www.savemyexams.com
Coloured Complexes CIE A Level Chemistry Revision Notes 2025 How Does Electron Promotion Work It can be gained or lost through molecular collisions and heat transfer. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. When an electron is promoted to an electronic excited state, it. How Does Electron Promotion Work.
From slidetodoc.com
Chapter 6 Electronic Structure of Atoms 6 1 How Does Electron Promotion Work Vibrational energy, however, is not exchanged exclusively by means of photons. It can be gained or lost through molecular collisions and heat transfer. The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination.. How Does Electron Promotion Work.
From www.science.org
Vibrational Promotion of Electron Transfer Science How Does Electron Promotion Work When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). Since electrons are indisinguishable and don't have specific. Vibrational energy, however, is not exchanged exclusively by means of photons. Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to. How Does Electron Promotion Work.
From www.numerade.com
SOLVED Show how the concept of electron promotion and orbital How Does Electron Promotion Work Vibrational energy, however, is not exchanged exclusively by means of photons. A key point is that electrons do not actually reside in specific orbitals. Since electrons are indisinguishable and don't have specific. Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Electron promotion is when. How Does Electron Promotion Work.
From www.semanticscholar.org
Figure 2 from Modeling hotelectron generation induced by electron How Does Electron Promotion Work Therefore, energy is actually released when carbon. In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that would be appropriate to promote an electron to a higher energy level. Vibrational energy, however, is not exchanged exclusively by means of photons. It can be gained or lost through molecular collisions and heat. How Does Electron Promotion Work.
From www.slideserve.com
PPT AS Chemistry PowerPoint Presentation, free download ID2093824 How Does Electron Promotion Work Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Since electrons are indisinguishable and don't have specific. A key point is that electrons do not actually reside in specific orbitals. Vibrational energy, however, is not exchanged exclusively by means of photons. The formation of hybrid. How Does Electron Promotion Work.
From education-portal.com
Electron Transitions & Spectral Lines Video & Lesson Transcript How Does Electron Promotion Work Since electrons are indisinguishable and don't have specific. It can be gained or lost through molecular collisions and heat transfer. A key point is that electrons do not actually reside in specific orbitals. Vibrational energy, however, is not exchanged exclusively by means of photons. Different wavelengths would be able to promote different electrons, depending on the energy difference between an. How Does Electron Promotion Work.
From www.slideserve.com
PPT Chapter 18 Electrical Properties PowerPoint Presentation, free How Does Electron Promotion Work The formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination. When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)).. How Does Electron Promotion Work.
From www.youtube.com
2.2 Excitation as Electron Promotion YouTube How Does Electron Promotion Work Since electrons are indisinguishable and don't have specific. Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence. It can be gained or lost through molecular collisions and heat transfer. Vibrational energy, however, is not exchanged exclusively by means of photons. A key point is that electrons. How Does Electron Promotion Work.
From mungfali.com
9.8 Molecular Orbital Theory Chemistry Libretexts 22B How Does Electron Promotion Work When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Therefore, energy is actually released when carbon. Vibrational energy, however, is not exchanged. How Does Electron Promotion Work.
From www.nagwa.com
Question Video Selecting the Term That Describes an Electron That Has How Does Electron Promotion Work When an electron is promoted to an electronic excited state, it often ends up in an excited vibrational state as well (figure \(\pageindex{4}\)). Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Vibrational energy, however, is not exchanged exclusively by means of photons. Since electrons. How Does Electron Promotion Work.
From www.slideserve.com
PPT Three Types of Bonds PowerPoint Presentation, free download ID How Does Electron Promotion Work Electron promotion is when an electron absorbs a photon to jump from a low energy level orbital to a higher energy orbital, hence. Since electrons are indisinguishable and don't have specific. Different wavelengths would be able to promote different electrons, depending on the energy difference between an occupied electronic energy level and an unoccupied one. Vibrational energy, however, is not. How Does Electron Promotion Work.
From www.researchgate.net
Electron promotion and fragmentation scheme of region A in Fig. 11 [6 How Does Electron Promotion Work In the case of visible or ultraviolet light, the energy of a photon is roughly in the region that would be appropriate to promote an electron to a higher energy level. Vibrational energy, however, is not exchanged exclusively by means of photons. Since electrons are indisinguishable and don't have specific. Therefore, energy is actually released when carbon. When an electron. How Does Electron Promotion Work.