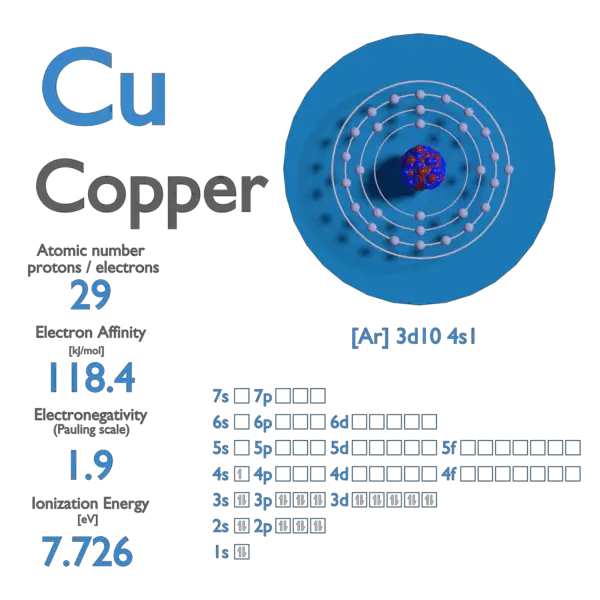
Copper In Electrons . Members of a group typically have similar properties and electron configurations in. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. A vertical column in the periodic table. The ground state electronic configuration of neutral copper is [ar]. It has symbol cu (from latin cuprum) and atomic number 29. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Therefore, the number of electrons in neutral atom of copper is 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. Copper is a chemical element; Copper atoms have 29 electrons and the shell structure is 2.8.18.1. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals.
from www.nuclear-power.com
The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. Therefore, the number of electrons in neutral atom of copper is 29. Members of a group typically have similar properties and electron configurations in. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Copper is a chemical element; Copper atoms have 29 electrons and the shell structure is 2.8.18.1. The ground state electronic configuration of neutral copper is [ar]. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. A vertical column in the periodic table.
Copper Electron Affinity Electronegativity Ionization Energy of
Copper In Electrons A vertical column in the periodic table. Therefore, the number of electrons in neutral atom of copper is 29. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. It has symbol cu (from latin cuprum) and atomic number 29. Members of a group typically have similar properties and electron configurations in. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The ground state electronic configuration of neutral copper is [ar]. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A vertical column in the periodic table. Copper is a chemical element; Copper atoms have 29 electrons and the shell structure is 2.8.18.1.
From www.numerade.com
The freeelectron density in a copper wire is 8.5 ×10^28 electrons / m Copper In Electrons It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Therefore, the number of electrons in neutral atom of copper is 29. Copper is a chemical element; A vertical. Copper In Electrons.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper In Electrons It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we. Copper In Electrons.
From valenceelectrons.com
How many valence electrons does aluminum(Al) have? Copper In Electrons Copper atoms have 29 electrons and the shell structure is 2.8.18.1. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. A vertical column in the periodic table. It has symbol cu (from latin cuprum) and atomic number 29. The ground state electronic configuration of neutral copper is [ar]. How to write the electron. Copper In Electrons.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper In Electrons The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in. The ground state electronic configuration of neutral copper is [ar]. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. How to write the. Copper In Electrons.
From www.alamy.com
Copper atom hires stock photography and images Alamy Copper In Electrons A vertical column in the periodic table. It has symbol cu (from latin cuprum) and atomic number 29. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron configuration of copper. Copper In Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper II Easy & Quick YouTube Copper In Electrons It has symbol cu (from latin cuprum) and atomic number 29. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. Copper is a chemical element; Therefore, the number of electrons in neutral atom of copper is 29. The ground state electronic configuration of neutral copper is [ar]. It is a soft, malleable, and ductile metal with very high. Copper In Electrons.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper In Electrons The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration. Copper In Electrons.
From www.nuclear-power.com
Copper Electron Affinity Electronegativity Ionization Energy of Copper In Electrons Therefore, the number of electrons in neutral atom of copper is 29. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. It has symbol cu (from latin cuprum) and atomic number 29. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. Members of a group typically have similar properties and electron. Copper In Electrons.
From awesomehome.co
Copper Periodic Table Protons Neutrons And Electrons Awesome Home Copper In Electrons How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. The ground state electronic configuration of neutral copper is [ar]. Therefore, the number of electrons in neutral atom of. Copper In Electrons.
From proper-cooking.info
Copper Electron Dot Diagram Copper In Electrons Members of a group typically have similar properties and electron configurations in. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. Therefore, the number of electrons in neutral atom of copper is 29. A vertical column in the periodic table. Copper is. Copper In Electrons.
From awesomehome.co
Copper Periodic Table Protons Neutrons And Electrons Awesome Home Copper In Electrons It has symbol cu (from latin cuprum) and atomic number 29. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. A vertical column in the periodic table. Therefore, the number of electrons. Copper In Electrons.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper In Electrons Copper is a chemical element; Therefore, the number of electrons in neutral atom of copper is 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The ground state electronic. Copper In Electrons.
From awesomehome.co
Copper Periodic Table Protons Neutrons And Electrons Awesome Home Copper In Electrons The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Members of a group typically have similar properties and electron configurations in. The ground state electronic configuration of neutral copper is [ar]. It has symbol cu (from latin cuprum) and atomic number 29. It is a. Copper In Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Copper (Cu Copper In Electrons It has symbol cu (from latin cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Therefore, the number of electrons in neutral atom of copper. Copper In Electrons.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper In Electrons How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. Members of a group typically have similar properties and electron configurations in. The electron distribution in copper spans across the k, l, m,. Copper In Electrons.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of Copper In Electrons Members of a group typically have similar properties and electron configurations in. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. A vertical column in the periodic table. Therefore, the number of electrons in neutral atom of copper is 29. It has symbol. Copper In Electrons.
From awesomehome.co
Copper Periodic Table Protons Neutrons And Electrons Awesome Home Copper In Electrons It has symbol cu (from latin cuprum) and atomic number 29. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. The electron distribution in copper spans across the k, l, m, and. Copper In Electrons.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Copper In Electrons Copper is a chemical element; The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. Members of a group typically have similar properties and electron configurations in. The ground state. Copper In Electrons.
From favpng.com
Bohr Model Electron Shell Copper Atom Valence Electron, PNG Copper In Electrons It has symbol cu (from latin cuprum) and atomic number 29. The ground state electronic configuration of neutral copper is [ar]. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. The electron distribution in copper spans across the. Copper In Electrons.
From www.earthdate.org
Copper’s Superpower EarthDate Copper In Electrons How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. A vertical column in the periodic table. Copper is a. Copper In Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Copper In Electrons A vertical column in the periodic table. The ground state electronic configuration of neutral copper is [ar]. Members of a group typically have similar properties and electron configurations in. Copper is a chemical element; The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. The electron distribution in copper spans across the k, l,. Copper In Electrons.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper In Electrons It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. It has symbol cu (from latin cuprum) and atomic number 29. The ground state electronic configuration of neutral copper is [ar]. Copper is a chemical element; The electron distribution. Copper In Electrons.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper In Electrons Copper is a chemical element; It has symbol cu (from latin cuprum) and atomic number 29. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in. The electron distribution in copper spans across the k, l, m, and n shells,. Copper In Electrons.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper In Electrons It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. It has symbol cu (from latin cuprum) and atomic number 29. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The ground state electronic configuration of neutral copper. Copper In Electrons.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Copper In Electrons The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. It has symbol cu (from latin cuprum) and atomic number 29. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know. Copper In Electrons.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Copper In Electrons Copper atoms have 29 electrons and the shell structure is 2.8.18.1. Members of a group typically have similar properties and electron configurations in. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Copper is a chemical element; Therefore, the number of electrons in neutral atom. Copper In Electrons.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Copper In Electrons Members of a group typically have similar properties and electron configurations in. The ground state electronic configuration of neutral copper is [ar]. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. It has symbol cu (from latin cuprum) and atomic number 29. Copper is a chemical element; The electron configuration of copper refers to. Copper In Electrons.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Periodic Table Element Comparison Compare Copper vs Aluminium Copper In Electrons Therefore, the number of electrons in neutral atom of copper is 29. Copper atoms have 29 electrons and the shell structure is 2.8.18.1. A vertical column in the periodic table. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. The electron distribution in copper spans across the k, l, m, and n shells, with. Copper In Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper In Electrons Copper atoms have 29 electrons and the shell structure is 2.8.18.1. A vertical column in the periodic table. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Members of a group typically have similar properties and electron configurations in. It is a soft,. Copper In Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper In Electrons Copper is a chemical element; The ground state electronic configuration of neutral copper is [ar]. A vertical column in the periodic table. Therefore, the number of electrons in neutral atom of copper is 29. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. Members of a group typically have similar properties and electron. Copper In Electrons.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper In Electrons Copper atoms have 29 electrons and the shell structure is 2.8.18.1. It has symbol cu (from latin cuprum) and atomic number 29. The ground state electronic configuration of neutral copper is [ar]. Members of a group typically have similar properties and electron configurations in. Copper is a chemical element; How to write the electron configuration for copper (cu, cu+, and. Copper In Electrons.
From socratic.org
Can you describe the process that releases electrons in a zinc copper Copper In Electrons Therefore, the number of electrons in neutral atom of copper is 29. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Members of a group typically have similar properties and electron configurations in. The electron distribution in copper spans across the k, l,. Copper In Electrons.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Copper In Electrons How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. A vertical column in the periodic table. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. It is a. Copper In Electrons.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper In Electrons The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. Copper is a chemical element; The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals.. Copper In Electrons.
From www.youtube.com
(a) Estimate the average drift speed of conduction electrons in a Copper In Electrons Copper atoms have 29 electrons and the shell structure is 2.8.18.1. Members of a group typically have similar properties and electron configurations in. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. The ground state electronic configuration of neutral copper is [ar]. It has symbol cu (from latin cuprum) and atomic number 29. How. Copper In Electrons.