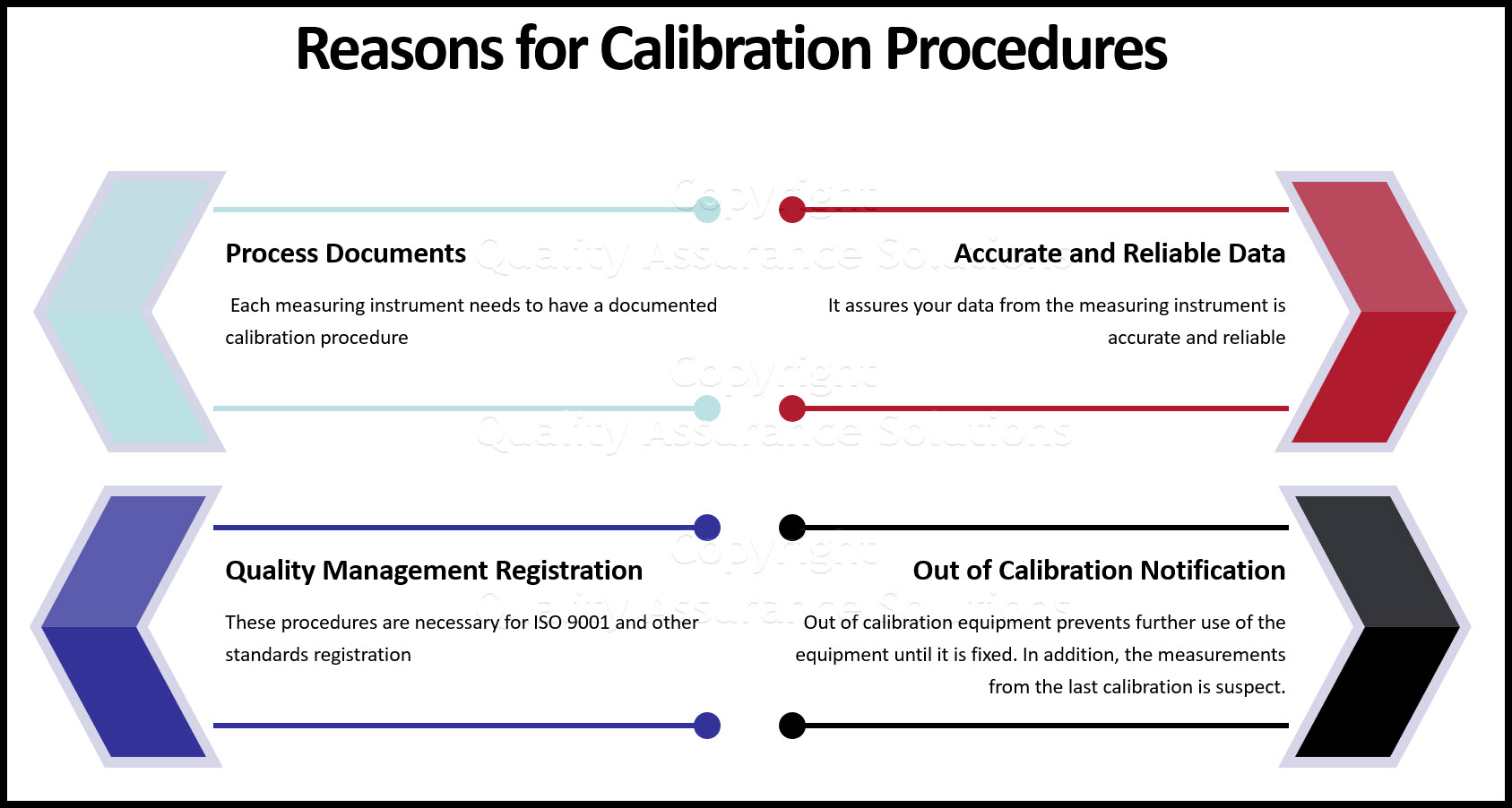
Quality Audit Calibration . Equip with the necessary skills to interpret and understand the content of a typical calibration reports. It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Identify and understand the common. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier.
from www.quality-assurance-solutions.com
The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Equip with the necessary skills to interpret and understand the content of a typical calibration reports. It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration. Identify and understand the common. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,.
Weight Scale Calibration Example
Quality Audit Calibration Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. Identify and understand the common. The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. Equip with the necessary skills to interpret and understand the content of a typical calibration reports. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration.
From www.bizmanualz.com
Calibration Log ISO Template Quality Audit Calibration Equip with the necessary skills to interpret and understand the content of a typical calibration reports. The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. It details how calibration activities should be performed, the responsibilities. Quality Audit Calibration.
From issuu.com
ISO 17025 Calibration Laboratory Documents by Global Manager Group Issuu Quality Audit Calibration A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. Identify and understand the common. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. It. Quality Audit Calibration.
From besttemplate-ideas.blogspot.com
Information System Audit Report Template Best Template Ideas Quality Audit Calibration It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. Identify and understand the common. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories). Quality Audit Calibration.
From alinksi.com
American links Quality Audit Calibration A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip. Quality Audit Calibration.
From www.vrogue.co
Call Center Scorecard Template Excel Template 2 Resum vrogue.co Quality Audit Calibration Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. Identify and understand the common. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so.. Quality Audit Calibration.
From za.pinterest.com
Browse Our Sample of Supplier Audit Checklist Template for Free Quality Audit Calibration The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. It details how calibration activities should be performed, the responsibilities for each task and any other conditions. Quality Audit Calibration.
From www.alamy.com
Quality control inspector line icons collection. Accuracy, Attention Quality Audit Calibration Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple. Quality Audit Calibration.
From gagetrak.com
ISO Audit Calibration Performance Report GAGEtrak Calibration Quality Audit Calibration It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration. The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Calibration concept,. Quality Audit Calibration.
From www.scribd.com
Calibration Format Report Instrumentation Calibration Quality Audit Calibration Identify and understand the common. Equip with the necessary skills to interpret and understand the content of a typical calibration reports. The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. A calibrated equipment procedure describes. Quality Audit Calibration.
From elsmar.com
Internal Audit Schedule Example Quality Audit Calibration Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. Identify and understand the common. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing,. Quality Audit Calibration.
From genicassets.com
Audits Calibration Management Software Genic Assets Quality Audit Calibration Equip with the necessary skills to interpret and understand the content of a typical calibration reports. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. This program is designed to. Quality Audit Calibration.
From carelabz.com
Calibration certificate 1 SAMPLE Carelabs Quality Audit Calibration This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Equip with the necessary skills to interpret and understand the content of a typical calibration. Quality Audit Calibration.
From certificationmalta.com
ISO 90012015 Internal Audit Criteria with examples Quality Audit Calibration Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. This program is designed to enable the. Quality Audit Calibration.
From forcetechnology.com
Highpressure calibration of flow meters Quality Audit Calibration Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. A calibrated equipment procedure describes. Quality Audit Calibration.
From genicassets.com
Audits Calibration Genic Assets site Quality Audit Calibration Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. It details how calibration activities should. Quality Audit Calibration.
From www.slideteam.net
GMP Audit Checklist To Address Organizational Responsibilities Quality Quality Audit Calibration Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Equip with the necessary skills to interpret and understand the content of a typical calibration reports. Identify and understand the common. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. Iso/iec 17025:2017 &. Quality Audit Calibration.
From www.bizmanualz.com
ISO Calibration Record ISO Template Quality Audit Calibration Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. Identify and understand the common. Iso/iec 17025:2017 & internal auditor training (general requirements for the. Quality Audit Calibration.
From www.quality-assurance-solutions.com
Tool Calibration and Control System Quality Audit Calibration Identify and understand the common. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. Equip with the necessary skills to interpret and understand the content of a typical calibration reports. A calibrated equipment procedure describes. Quality Audit Calibration.
From studylib.net
HIACflyercalibrationservices Quality Audit Calibration Equip with the necessary skills to interpret and understand the content of a typical calibration reports. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. This program is designed to enable the participants. Quality Audit Calibration.
From instrumentationbasic.com
Instrument Calibration Report Instrumentation basics Quality Audit Calibration Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. The audit process is based on external auditing (typically replicating the conformity assessment body (cab). Quality Audit Calibration.
From theinstrumentguru.com
Calibration Certificate Quality Audit Calibration It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. Calibration audit checklist gage. Quality Audit Calibration.
From mavink.com
Contoh Format Audit Quality Audit Calibration The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. This program is designed. Quality Audit Calibration.
From www.bizmanualz.com
AS9100 Calibration Record Quality Audit Calibration Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration. Quality Audit Calibration.
From old.sermitsiaq.ag
Quality Audit Template Quality Audit Calibration It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. Iso/iec 17025:2017 & internal. Quality Audit Calibration.
From waremaz.weebly.com
Free iso 9001 2015 audit checklist excel xls waremaz Quality Audit Calibration This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Calibration audit checklist gage identification unique identifier for every measurement and test device type of. Quality Audit Calibration.
From ukitnetworks.com
Why Calibration is Essential in Quality Monitoring UK IT Networks Quality Audit Calibration This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. Identify and understand the common. Whether you’re undergoing iso, fda, or customer audits, calibration is. Quality Audit Calibration.
From genicassets.com
Audits Calibration Management Software Genic Assets Quality Audit Calibration Equip with the necessary skills to interpret and understand the content of a typical calibration reports. The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. This program is designed to enable the. Quality Audit Calibration.
From www.inpaspages.com
Daily Calibration Report Quality Audit Calibration This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and controlling calibration activities carried out for inspection,. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. Equip with the necessary skills to interpret and understand the content of a typical calibration. Quality Audit Calibration.
From www.researchgate.net
(PDF) Internal Audit Checklist QMS ISO 90012015 Quality Audit Calibration The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. This program is designed to enable the. Quality Audit Calibration.
From medicaldeviceacademy.com
Improving Your ISO Internal Auditing Schedule Quality Audit Calibration The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. Calibration concept, iso 9001 requirements & common issues found in calibration explained in a simple manner so. A calibrated equipment procedure describes the process. Quality Audit Calibration.
From www.quality-assurance-solutions.com
Weight Scale Calibration Example Quality Audit Calibration Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. Equip with the necessary skills to interpret and understand the content of a typical calibration reports. Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. This program is designed to enable the participants. Quality Audit Calibration.
From www.scribd.com
Supplier Audit Check Sheet PDF Calibration Quality Management System Quality Audit Calibration Identify and understand the common. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. Iso/iec 17025:2017 & internal auditor training (general requirements for the competence of testing and calibration laboratories) to equip the. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising and. Quality Audit Calibration.
From www.template.net
13+ Quality Audit Report Templates Google Docs, Word, Pages, PDF Quality Audit Calibration The audit process is based on external auditing (typically replicating the conformity assessment body (cab) or supplier. Calibration audit checklist gage identification unique identifier for every measurement and test device type of gage e.g. A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. Iso/iec 17025:2017 & internal auditor training. Quality Audit Calibration.
From www.inpaspages.com
Calibration Status Verification Report format Quality Audit Calibration A calibrated equipment procedure describes the process that tests and records the accuracy of all measuring, testing, and monitoring equipment. It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration. This program is designed to enable the participants to acquire the practical knowledge and skills in identifying, organising. Quality Audit Calibration.
From www.cooperfluidsystems.com.au
Cooper Fluid Systems Mobile Audit and Calibration Quality Audit Calibration Whether you’re undergoing iso, fda, or customer audits, calibration is a critical component of maintaining compliance and ensuring quality. It details how calibration activities should be performed, the responsibilities for each task and any other conditions or limitations to the calibration. Equip with the necessary skills to interpret and understand the content of a typical calibration reports. The audit process. Quality Audit Calibration.