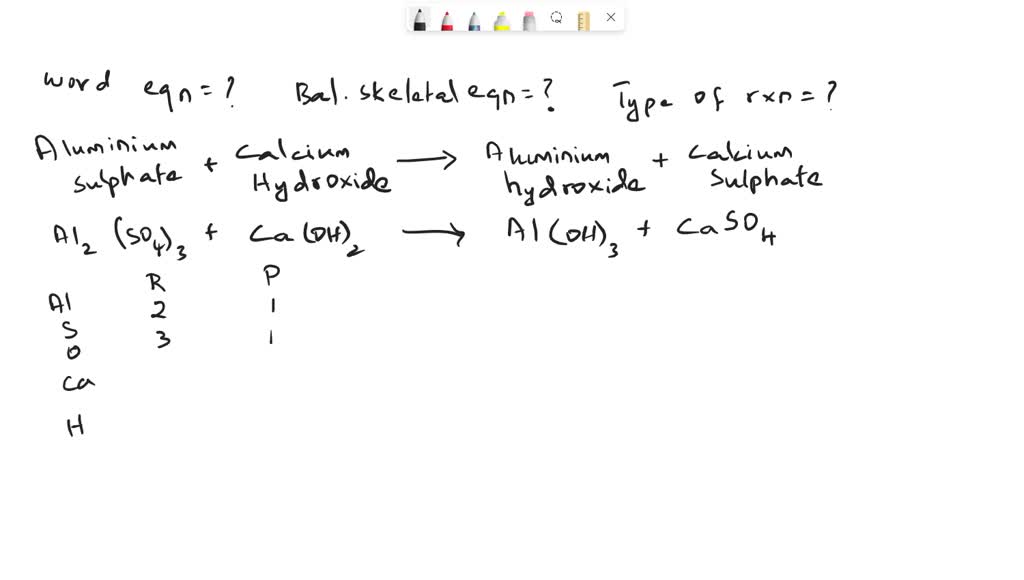
Aluminum Sulfate Reacts With Sodium Hydroxide . Write the balanced chemical equations of the following reactions. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide) in this video we'll balance the. This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. 2 al 3 + + 6. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: (b) potassium bicarbonate + sulphuric acid →. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2.
from www.numerade.com
Write the balanced chemical equations of the following reactions. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide) in this video we'll balance the. 2 al 3 + + 6. (b) potassium bicarbonate + sulphuric acid →. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water.
SOLVED Aluminum sulfate solution and calcium hydroxide solution
Aluminum Sulfate Reacts With Sodium Hydroxide 2 al 3 + + 6. Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide) in this video we'll balance the. This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). (b) potassium bicarbonate + sulphuric acid →. Write the balanced chemical equations of the following reactions. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. 2 al 3 + + 6.
From www.numerade.com
SOLVEDAqueous solutions of aluminum chloride and sodium hydroxide are Aluminum Sulfate Reacts With Sodium Hydroxide How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide) in this video we'll balance the. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. Write the balanced chemical equations of the following reactions.. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.thesciencehive.co.uk
Identifying Ions (AQA) — the science sauce Aluminum Sulfate Reacts With Sodium Hydroxide Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. (b) potassium bicarbonate + sulphuric acid →. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
What happens when aluminum sulfate (Al2(SO4)3) react with sodium Aluminum Sulfate Reacts With Sodium Hydroxide A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. Write the balanced chemical equations of the following reactions. This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). Al2(so4)3 + naoh. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Determine if the following reactions will form a precipitate Aluminum Sulfate Reacts With Sodium Hydroxide Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. Write the balanced chemical equations of the following reactions. (b) potassium bicarbonate + sulphuric acid →. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. This video is a practical demonstration of the reaction of. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.slideshare.net
Sodium hydroxide industrial use Aluminum Sulfate Reacts With Sodium Hydroxide (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Write the balanced chemical equations of the following reactions. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. How to balance al2 (so4)3 + naoh = al (oh)3 +. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Aluminum Sulfate Reacts With Sodium Hydroxide When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. Write the balanced chemical equations of the following reactions. Al2(so4)3 + naoh = al(oh)3 +. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.sciencephoto.com
Aluminium Sulphate added to Sodium Carbonate, 1 of 3 Stock Image C030 Aluminum Sulfate Reacts With Sodium Hydroxide Write the balanced chemical equations of the following reactions. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: 2 al 3 + + 6. When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Al 2 (so. Aluminum Sulfate Reacts With Sodium Hydroxide.
From byjus.com
The balanced net ionic equation for the reaction of aluminium sulphate Aluminum Sulfate Reacts With Sodium Hydroxide This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: Write the balanced chemical equations of the following reactions. Al2(so4)3. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.mdpi.com
Applied Sciences Free FullText Features of the Hydrosulfate Method Aluminum Sulfate Reacts With Sodium Hydroxide A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. (b) potassium bicarbonate + sulphuric acid →. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. Al2(so4)3 + naoh = al(oh)3 + na2so4 is. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.tessshebaylo.com
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo Aluminum Sulfate Reacts With Sodium Hydroxide This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). 2 al 3 + + 6. Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. Write the balanced chemical equations of the following reactions. Al 2 (so 4) 3. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED a) Write the balanced chemical reaction for the following (2 Aluminum Sulfate Reacts With Sodium Hydroxide (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. 2 al 3 + + 6. When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: (b) potassium bicarbonate + sulphuric acid →. How to balance al2 (so4)3. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.teachoo.com
Chemical Reaction Definition, Types and Examples Class 10 Science Aluminum Sulfate Reacts With Sodium Hydroxide Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: (b) potassium bicarbonate +. Aluminum Sulfate Reacts With Sodium Hydroxide.
From exozjazrx.blob.core.windows.net
Aluminum Sulfate Reacts With Ammonium Hydroxide To Form at Julia Graig blog Aluminum Sulfate Reacts With Sodium Hydroxide Write the balanced chemical equations of the following reactions. Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide). Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Aluminum Sulfate Reacts With Sodium Hydroxide Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. A). Aluminum Sulfate Reacts With Sodium Hydroxide.
From socratic.org
What is the net ionic equation for the reaction "NaOH" + "Cu"("NO"_3)_2 Aluminum Sulfate Reacts With Sodium Hydroxide This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.sciencephoto.com
Aluminium precipitate in sodium hydroxide Stock Image C019/9198 Aluminum Sulfate Reacts With Sodium Hydroxide Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). Write the balanced chemical equations of the following reactions. 2 al 3 + + 6. Al 2 (so 4) 3. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.researchgate.net
(PDF) The Effects of Aluminium Sulphate on Slag Paste Activated with Aluminum Sulfate Reacts With Sodium Hydroxide How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide) in this video we'll balance the. Write the balanced chemical equations of the following reactions. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis). Aluminum Sulfate Reacts With Sodium Hydroxide.
From loenquyfr.blob.core.windows.net
Aluminum Sulfate And Sodium Hydroxide at Ernest blog Aluminum Sulfate Reacts With Sodium Hydroxide (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. Write the balanced chemical equations of the following reactions. Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. (b) potassium bicarbonate + sulphuric acid. Aluminum Sulfate Reacts With Sodium Hydroxide.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Aluminum Sulfate Reacts With Sodium Hydroxide Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Al. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Aluminum sulfate solution and calcium hydroxide solution Aluminum Sulfate Reacts With Sodium Hydroxide (b) potassium bicarbonate + sulphuric acid →. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.chegg.com
Solved Write A Balanced Chemical Equation For Each Of The... Aluminum Sulfate Reacts With Sodium Hydroxide A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: 2 al 3 + + 6. (b) potassium bicarbonate + sulphuric acid →. Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide. Aluminum Sulfate Reacts With Sodium Hydroxide.
From mariabsmitho.blob.core.windows.net
Lead Hydroxide Reaction Equation at mariabsmitho blog Aluminum Sulfate Reacts With Sodium Hydroxide This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). Write the balanced chemical equations of the following reactions. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. A) the balanced net ionic. Aluminum Sulfate Reacts With Sodium Hydroxide.
From brainly.in
what do you observe when ammonium hydroxide is added to iron(II Aluminum Sulfate Reacts With Sodium Hydroxide Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. Write the balanced chemical equations of the following reactions. Al 2 (so. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.toppr.com
The balanced equation for the following reactionCopper sulphate Aluminum Sulfate Reacts With Sodium Hydroxide Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. Write the balanced chemical equations of the following reactions. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide) in this video we'll balance the. This video is. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Aluminium sulphate reacts with sodium hydroxide to from a Aluminum Sulfate Reacts With Sodium Hydroxide Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). When aluminium sulfate reacts with an. Aluminum Sulfate Reacts With Sodium Hydroxide.
From loenquyfr.blob.core.windows.net
Aluminum Sulfate And Sodium Hydroxide at Ernest blog Aluminum Sulfate Reacts With Sodium Hydroxide (b) potassium bicarbonate + sulphuric acid →. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. When aluminium sulfate reacts with an alkaline hydroxide, such as. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
How to Balance Al2(SO4)3 + NaOH = Al(OH)3 + Na2SO4 (Aluminum sulfate Aluminum Sulfate Reacts With Sodium Hydroxide How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide) in this video we'll balance the. When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: Write the balanced chemical equations of the following reactions. Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium. Aluminum Sulfate Reacts With Sodium Hydroxide.
From aluminumsulfatepitsukata.blogspot.com
Aluminum Sulfate Reaction Of Aluminum Sulfate With Ammonia Aluminum Sulfate Reacts With Sodium Hydroxide Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. (b) potassium bicarbonate + sulphuric acid →. 2 al 3 + + 6. Al 2 (so 4) 3 (s) + 6 naoh (aq) → 2. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: (a) sodium. Aluminum Sulfate Reacts With Sodium Hydroxide.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Reaction Of Aluminium Oxide With Sulphuric Acid Aluminum Sulfate Reacts With Sodium Hydroxide (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Write the balanced chemical equations of the following reactions. When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: 2 al 3 + + 6. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: Find the standard. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Aluminum nitrate reacts with sodium hydroxide to form a Aluminum Sulfate Reacts With Sodium Hydroxide A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Write the balanced chemical equations of the following reactions. Al2(so4)3 + naoh = al(oh)3 + na2so4. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
How to Balance FeCl3 + Na2S = Fe2S3 + NaCl (Iron (III) chloride Aluminum Sulfate Reacts With Sodium Hydroxide Write the balanced chemical equations of the following reactions. When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide) in this video we'll balance the. Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Aluminum Sulfate Reacts With Sodium Hydroxide When aluminium sulfate reacts with an alkaline hydroxide, such as sodium hydroxide, aluminium hydroxide precipitates: Find the standard enthalpy of reaction between solid aluminum sulfate and aqueous sodium hydroxide. 2 al 3 + + 6. (b) potassium bicarbonate + sulphuric acid →. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Al2(so4)3 + naoh = al(oh)3 + na2so4. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED write the balanced equation for each of the reactions 7 Sodium Aluminum Sulfate Reacts With Sodium Hydroxide This video is a practical demonstration of the reaction of aluminum sulfate (al2 (so4)3) & sodium hydroxide (naoh). 2 al 3 + + 6. (a) sodium hydroxide + sulphuric acid → sodium sulphate + water. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. Al 2 (so 4) 3 (s) + 6. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
Aluminum Sulfate And Calcium Hydroxide Make Aluminum Hydroxide And Aluminum Sulfate Reacts With Sodium Hydroxide Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium hydroxide is: Al2(so4)3 + naoh = al(oh)3 + na2so4 is a double displacement (metathesis) reaction where one mole of aqueous aluminum sulfate [al 2. Al 2 (so 4) 3. Aluminum Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
What happens when Barium chloride (BaCl2) reacts with Aluminum sulphate Aluminum Sulfate Reacts With Sodium Hydroxide 2 al 3 + + 6. How to balance al2 (so4)3 + naoh = al (oh)3 + na2so4 (aluminum sulfate + sodium hydroxide) in this video we'll balance the. Al 2 (so 4) 3 + 3 naoh → 2 al (oh) 3 + 3/2 na 2. A) the balanced net ionic equation for the reaction between aluminum sulfate and sodium. Aluminum Sulfate Reacts With Sodium Hydroxide.