Blocks Vs Periods . A period is a horizontal row of elements on the periodic table. Are arranged in the periodic table. Let us discuss them one by one. The horizontal rows are called periods. Periods of the modern periodic table. Here are the main features of the table: For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period. First period contain only two elements that is. There are 7 periods in the periodic table. Groups and periods are two ways of categorizing elements in the periodic table.
from coppellstudentmedia.com
Let us discuss them one by one. Groups and periods are two ways of categorizing elements in the periodic table. A period is a horizontal row of elements on the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Groups and periods organize elements on the periodic table of the elements. Here are the main features of the table: Atomic number increases as you move down a group or across a period. Are arranged in the periodic table. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The horizontal rows are called periods.
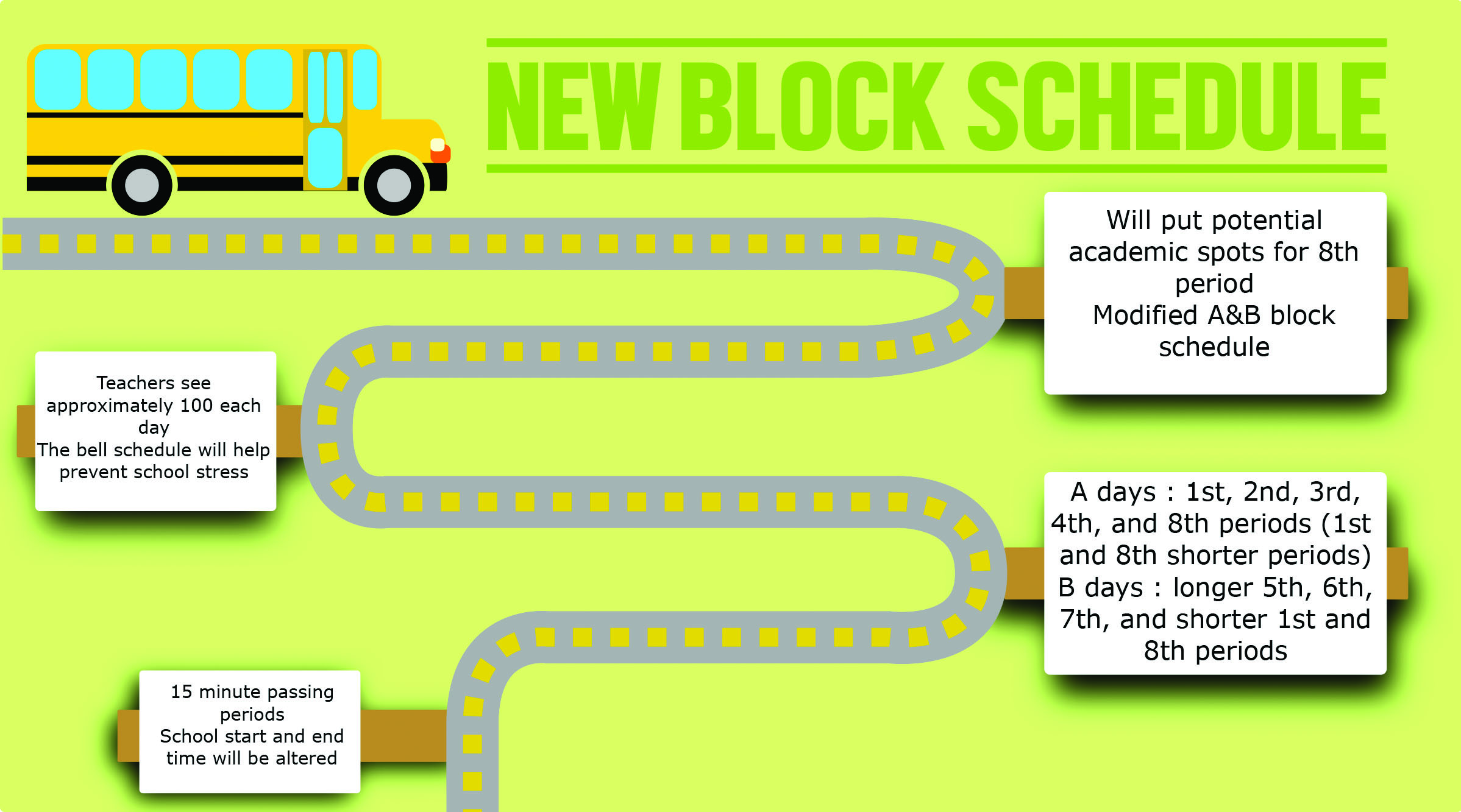
Coppell Student Media Block scheduling in Coppell’s future
Blocks Vs Periods Let us discuss them one by one. There are 7 periods in the periodic table. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Here are the main features of the table: Groups and periods organize elements on the periodic table of the elements. Are arranged in the periodic table. A periodic table group is a column, while a periodic table period is a row. Atomic number increases as you move down a group or across a period. Groups and periods are two ways of categorizing elements in the periodic table. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Let us discuss them one by one. Periods of the modern periodic table. First period contain only two elements that is. The horizontal rows are called periods. A period is a horizontal row of elements on the periodic table.
From www.facebook.com
Yellow Jackets vs Cougars Full Game Yellow Jackets vs Cougars Blocks Vs Periods Periods of the modern periodic table. Atomic number increases as you move down a group or across a period. Groups and periods are two ways of categorizing elements in the periodic table. A period is a horizontal row of elements on the periodic table. Here are the main features of the table: Groups and periods organize elements on the periodic. Blocks Vs Periods.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Blocks Vs Periods The horizontal rows are called periods. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Atomic number increases as you move down a group or across a period. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Are arranged in the periodic table. First period contain only two elements that is. There. Blocks Vs Periods.
From mehdiperiodictable.weebly.com
Introduction The Periodic Table Blocks Vs Periods First period contain only two elements that is. Atomic number increases as you move down a group or across a period. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Here are the main features of the table: The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Periods of the modern periodic. Blocks Vs Periods.
From www.pinterest.com
New Periodic Table Groups Vs Periods tablepriodic priodic Blocks Vs Periods Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. First period contain only two elements that is. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The horizontal rows are called periods. Groups and periods are two ways of categorizing elements in the periodic table. Let us discuss. Blocks Vs Periods.
From www.powersystemsdesign.com
Using SPICE model blocks Part Three Blocks Vs Periods Here are the main features of the table: Let us discuss them one by one. The horizontal rows are called periods. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. A periodic table group is a column, while a periodic table period is a row. There are 7 periods in the periodic table.. Blocks Vs Periods.
From mungfali.com
Implantation Bleeding Versus Period Blocks Vs Periods First period contain only two elements that is. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Groups and periods organize elements on the periodic table of the elements. The horizontal rows are called periods. A period is a horizontal row of elements on the periodic table. There are 7 periods in the periodic table. Let us. Blocks Vs Periods.
From nypost.com
Exclusive Crime down along longtroubled NYC block City Hall Blocks Vs Periods Are arranged in the periodic table. First period contain only two elements that is. Atomic number increases as you move down a group or across a period. Periods of the modern periodic table. Groups and periods organize elements on the periodic table of the elements. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The. Blocks Vs Periods.
From traveljula.blogspot.com
Arkansas Traveler Quilt Block traveljula Blocks Vs Periods Periods of the modern periodic table. Here are the main features of the table: Atomic number increases as you move down a group or across a period. A periodic table group is a column, while a periodic table period is a row. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. First period. Blocks Vs Periods.
From nypost.com
Exclusive Crime down along longtroubled NYC block City Hall Blocks Vs Periods For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. A period is a horizontal row of elements on the periodic table. Here are the main features of the table: The horizontal rows are called periods. Let us discuss them one by one. Atomic number increases as you move down a group or across a period.. Blocks Vs Periods.
From leecountytoday.blogspot.com
Periodic Table Groups And Periods Decoration For Wedding Blocks Vs Periods A period is a horizontal row of elements on the periodic table. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. First period contain only two elements that is. Are arranged in the periodic table. Let us discuss them one by one. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Groups. Blocks Vs Periods.
From www.galvnews.com
Pius Suter scores twice as Canucks beat Sharks 32 Sports The Daily Blocks Vs Periods The horizontal rows are called periods. Groups and periods are two ways of categorizing elements in the periodic table. First period contain only two elements that is. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Atomic number increases as you move down a group or across a period. Groups and periods organize elements on the periodic. Blocks Vs Periods.
From academic-accelerator.com
周期表 Periodic Table 最新の百科事典、ニュース、レビュー、研究 Blocks Vs Periods Are arranged in the periodic table. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. A periodic table group is a column, while a periodic table period is a row. The horizontal rows are called periods. First period contain only two elements that is. A period is a horizontal row of elements on the periodic table. Periods. Blocks Vs Periods.
From byjus.com
Two blocks each of mass m connected by an ideal spring are placed over Blocks Vs Periods The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Let us discuss them one by one. A periodic table group is a column, while a periodic table period is a row. Atomic number increases as you move down a group or across a period. Here are the main features of the table: The horizontal rows are called. Blocks Vs Periods.
From sciencenotes.org
Periodic Table Groups and Periods Blocks Vs Periods Groups and periods organize elements on the periodic table of the elements. Are arranged in the periodic table. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a. Blocks Vs Periods.
From www.hotrod.com
BigBlock vs. SmallBlock V8 What’s the Difference? Blocks Vs Periods First period contain only two elements that is. Groups and periods are two ways of categorizing elements in the periodic table. There are 7 periods in the periodic table. Here are the main features of the table: Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. The horizontal rows are called periods. For. Blocks Vs Periods.
From www.facebook.com
Yellow Jackets vs Cougars Full Game Yellow Jackets vs Cougars Blocks Vs Periods The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Let us discuss them one by one. A periodic table group is a column, while a periodic table period is a row. Periods of the modern periodic table. Are arranged in the periodic table. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3.. Blocks Vs Periods.
From utedzz.blogspot.com
Periodic Table Periods Vs Groups Periodic Table Timeline Blocks Vs Periods For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. A period is a horizontal row of elements on the periodic table. Groups and periods are two ways of categorizing elements in the periodic table. The elements astatine (\(\ce{at}\)) and. Blocks Vs Periods.
From study.com
The Periodic Table Properties of Groups and Periods Video & Lesson Blocks Vs Periods Periods of the modern periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period. First period contain only two elements that is. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Groups and periods organize elements. Blocks Vs Periods.
From chemistryp2t7.pbworks.com
chemistryp2t7 / The Periodic Table of Elements Blocks Vs Periods There are 7 periods in the periodic table. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both. Blocks Vs Periods.
From www.revimage.org
Similarities And Differences Between Alkali Alkaline Earth Metals The Blocks Vs Periods A periodic table group is a column, while a periodic table period is a row. Here are the main features of the table: The horizontal rows are called periods. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Let us discuss them one by one. A period is a horizontal row of elements on the. Blocks Vs Periods.
From www.news.com.au
The Block star’s ‘terrifying’ oncamera panic attack Blocks Vs Periods Groups and periods organize elements on the periodic table of the elements. Periods of the modern periodic table. Let us discuss them one by one. The horizontal rows are called periods. Here are the main features of the table: For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Atomic number increases as you move down. Blocks Vs Periods.
From reviewhomedecor.co
2 The Horizontal Rows On Periodic Table Are Called Review Home Decor Blocks Vs Periods The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. A period is a horizontal row of elements on the periodic table. The horizontal rows are called periods. Periods of the modern periodic table. Groups and periods are two ways of categorizing elements in the periodic table. First period contain only two elements that is. There are 7. Blocks Vs Periods.
From www.slideserve.com
PPT Periodic Table Study Guide Determining Shells and Valence Blocks Vs Periods First period contain only two elements that is. Let us discuss them one by one. Groups and periods organize elements on the periodic table of the elements. A period is a horizontal row of elements on the periodic table. A periodic table group is a column, while a periodic table period is a row. Atomic number increases as you move. Blocks Vs Periods.
From reviewhomedecor.co
Names Of Some Groups In The Periodic Table Review Home Decor Blocks Vs Periods For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Are arranged in the periodic table. A periodic table group is a column, while a periodic table period is a row. There are 7 periods in the periodic table. Let us discuss them one by one. Atomic number increases as you move down a group or. Blocks Vs Periods.
From www.slideserve.com
PPT HMJB 2 Associates PowerPoint Presentation, free download ID27158 Blocks Vs Periods There are 7 periods in the periodic table. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. First period contain only two elements that is. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Groups and. Blocks Vs Periods.
From twobirdsfourhands.com
What Is The Difference Between A Group And Period On Periodic Table Of Blocks Vs Periods Periods of the modern periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. A period is a horizontal row of elements on the periodic table. There are 7 periods in the periodic table. First period contain only two elements that is. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in. Blocks Vs Periods.
From mavink.com
Implantation Bleeding Chart Blocks Vs Periods There are 7 periods in the periodic table. Let us discuss them one by one. Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. A period is a horizontal row of elements on the periodic table. A periodic table group. Blocks Vs Periods.
From sciencenotes.org
Periodic Table Blocks of Elements Blocks Vs Periods Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Here are the main features of the table: The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. A period is a horizontal row of elements on the periodic table. Let us discuss them one by one. Groups and periods are two. Blocks Vs Periods.
From giobngmpe.blob.core.windows.net
How Long Does It Take For Pregnancy Pills To Work at Audrey Lovejoy blog Blocks Vs Periods Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Groups and periods are two ways of categorizing elements in the periodic table. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. A periodic table group is. Blocks Vs Periods.
From www.facebook.com
Yellow Jackets vs Cougars Full Game Yellow Jackets vs Cougars Blocks Vs Periods There are 7 periods in the periodic table. First period contain only two elements that is. Are arranged in the periodic table. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Groups and periods organize elements on the periodic table of the elements. Atomic number increases as you move down a group or across a. Blocks Vs Periods.
From medium.com
Block Your Time, Period The Shortform Medium Blocks Vs Periods Are arranged in the periodic table. Atomic number increases as you move down a group or across a period. Groups and periods are two ways of categorizing elements in the periodic table. A periodic table group is a column, while a periodic table period is a row. There are 7 periods in the periodic table. The horizontal rows are called. Blocks Vs Periods.
From www.slideshare.net
The Periodic Table Blocks Vs Periods A period is a horizontal row of elements on the periodic table. Here are the main features of the table: Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. For example, the elements sodium (\(\ce{na}\)) and magnesium (\(\ce{mg}\)) are both in period 3. Let us discuss them one by one. There are 7. Blocks Vs Periods.
From pediaa.com
Difference Between S and P Block Elements Definition, Characteristic Blocks Vs Periods Are arranged in the periodic table. A periodic table group is a column, while a periodic table period is a row. Groups and periods organize elements on the periodic table of the elements. Here are the main features of the table: First period contain only two elements that is. The horizontal rows are called periods. Periods of the modern periodic. Blocks Vs Periods.
From coppellstudentmedia.com
Coppell Student Media Block scheduling in Coppell’s future Blocks Vs Periods Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Are arranged in the periodic table. Let us discuss them one by one. Atomic number increases as you move down a group or across a period. A period is a horizontal row of elements on the periodic table. A periodic table group is a. Blocks Vs Periods.
From www.chemistrylearner.com
Periodic Table Periods, Groups, and Families Blocks Vs Periods A periodic table group is a column, while a periodic table period is a row. The elements astatine (\(\ce{at}\)) and radon (\(\ce{rn}\)) are both in period 6. Periods of the modern periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Are arranged in the periodic table. Groups and periods organize elements. Blocks Vs Periods.