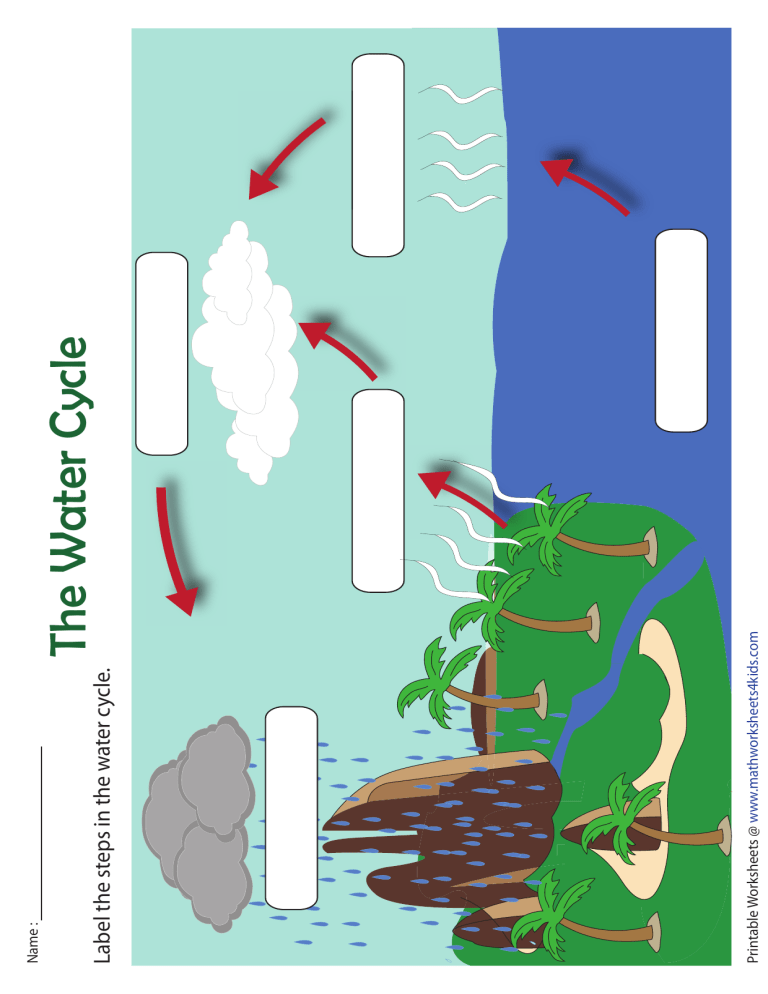
What Are The Components Of Water Answer Key . A solution with a ph of more than 7. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Identify that water is a compound common to living things. Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. Because of the higher electronegativity of the oxygen. A compound that prevents sharp,. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.
from studylib.net
Because of the higher electronegativity of the oxygen. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. A solution with a ph of more than 7. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. Identify that water is a compound common to living things. A compound that prevents sharp,. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)?
watercycleandanswerkeyworksheet3
What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? Because of the higher electronegativity of the oxygen. Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: A solution with a ph of more than 7. A compound that prevents sharp,. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Identify that water is a compound common to living things. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water.
From www.studocu.com
5 Properties of WaterS Properties of 1 Oxygen Covalent Hydrogen Hydrogen = electron presence What Are The Components Of Water Answer Key Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen. A compound that prevents sharp,. A solution with a ph of more than 7. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension. What Are The Components Of Water Answer Key.
From educational.my.id
Properties Of Water Worksheet Biology Educational.my.id What Are The Components Of Water Answer Key A solution with a ph of more than 7. Identify that water is a compound common to living things. Because of the higher electronegativity of the oxygen. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water,. What Are The Components Of Water Answer Key.
From quizzschoolhesitated.z21.web.core.windows.net
Properties Of Water Answer What Are The Components Of Water Answer Key As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. Because of the higher electronegativity of the oxygen. A solution with a ph of more than 7. This section describes the properties of water in the liquid and solid states and explains how. What Are The Components Of Water Answer Key.
From my-unit-property.netlify.app
Property Of Water Worksheet Answer Key What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.. What Are The Components Of Water Answer Key.
From oxazepamultramtki.blogspot.com
40 properties of water what makes water so special worksheet answers Worksheet For Fun What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? Because of the higher electronegativity of the oxygen. Identify that water is a compound common to living things. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure. What Are The Components Of Water Answer Key.
From studylib.net
ANSWER KEY Water water everywhere What Are The Components Of Water Answer Key Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Because of the higher electronegativity of the oxygen. A solution with a ph of more than 7. A compound that prevents sharp,. Identify that water is a compound common to living things. As water molecules make hydrogen bonds with each other, water. What Are The Components Of Water Answer Key.
From lessonmagiccoaching.z21.web.core.windows.net
Properties Of Water Questions And Answers What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? A compound that prevents sharp,. Identify that water is a compound common to living things. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Water is a simple molecule consisting of one oxygen atom. What Are The Components Of Water Answer Key.
From studylib.net
Properties of Water Parts I & II What Are The Components Of Water Answer Key This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? Identify that water is a compound common to living things. Because water seems so ubiquitous, many. What Are The Components Of Water Answer Key.
From www.housview.com
Printables Of Properties Of Water Worksheet Answers Free Worksheets Samples What Are The Components Of Water Answer Key Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A compound that prevents sharp,. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. A solution with a ph of more than 7. Because water seems. What Are The Components Of Water Answer Key.
From martindxmguide.blogspot.com
33 Properties Of Water Worksheet Answer Key support worksheet What Are The Components Of Water Answer Key Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high.. What Are The Components Of Water Answer Key.
From slideplayer.com
The importance of Water Quality and Testing Water ppt download What Are The Components Of Water Answer Key A compound that prevents sharp,. A solution with a ph of more than 7. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because water seems. What Are The Components Of Water Answer Key.
From www.worksheetsgo.com
The Water Cycle Worksheets WorksheetsGO What Are The Components Of Water Answer Key Because of the higher electronegativity of the oxygen. A solution with a ph of more than 7. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of. What Are The Components Of Water Answer Key.
From printableschooljedburgh.z21.web.core.windows.net
Properties Of Water Answer Key What Are The Components Of Water Answer Key Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water,. What Are The Components Of Water Answer Key.
From studylib.net
Properties of Water Worksheet Key What Are The Components Of Water Answer Key As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. Why is water able to form. What Are The Components Of Water Answer Key.
From learningfullherman.z19.web.core.windows.net
Properties Of Water Worksheet Answer Key What Are The Components Of Water Answer Key As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. A solution with a ph of. What Are The Components Of Water Answer Key.
From learningcognize.z13.web.core.windows.net
Properties Of Water Answer What Are The Components Of Water Answer Key Identify that water is a compound common to living things. Because of the higher electronegativity of the oxygen. A solution with a ph of more than 7. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. This section describes the properties of. What Are The Components Of Water Answer Key.
From zipworksheet.com
Properties Of Water Worksheet Biology What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? A compound that prevents sharp,. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Because of the higher electronegativity of the oxygen. A solution with a ph of more than 7. This section describes. What Are The Components Of Water Answer Key.
From dbdalrymplelethargy.z21.web.core.windows.net
Properties Of Water Biology Notes What Are The Components Of Water Answer Key Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Identify that water is a compound common to living things. Because of the higher electronegativity of the oxygen. A solution with a ph of more than. What Are The Components Of Water Answer Key.
From www.worksheetsgo.com
Water Cycle Diagram Worksheets WorksheetsGO What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? A compound that prevents sharp,. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: This section describes the. What Are The Components Of Water Answer Key.
From studylib.net
Water Cycle Notes What Are The Components Of Water Answer Key Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of. What Are The Components Of Water Answer Key.
From martindxmguide.blogspot.com
33 Properties Of Water Worksheet Answer Key support worksheet What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? Because of the higher electronegativity of the oxygen. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. A solution with a ph of more than. What Are The Components Of Water Answer Key.
From lessonlibraryfrivol.z21.web.core.windows.net
Properties Of Water Explained What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. A compound that prevents sharp,. A solution with a ph of more than 7. This section. What Are The Components Of Water Answer Key.
From educational.my.id
Properties Of Water Worksheet Biology Educational.my.id What Are The Components Of Water Answer Key This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. A compound that prevents sharp,. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: As water molecules make hydrogen bonds with each other, water. What Are The Components Of Water Answer Key.
From www.studocu.com
Phases of Water Lab Gizmo lab Name Date Student Exploration Phases of Water Vocabulary What Are The Components Of Water Answer Key Identify that water is a compound common to living things. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: A compound that prevents sharp,. Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? Because of the higher electronegativity of the oxygen. A solution. What Are The Components Of Water Answer Key.
From studylib.net
Study Guide for Water and Its Properties Test What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A solution with a ph of more than 7. Because of the higher electronegativity of the oxygen. As water molecules make hydrogen bonds with each other, water. What Are The Components Of Water Answer Key.
From mavink.com
Phases Of Water Cycle What Are The Components Of Water Answer Key A compound that prevents sharp,. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: A solution with a ph of more than 7. Because. What Are The Components Of Water Answer Key.
From www.studypool.com
SOLUTION Ce 104 key components of water distribution system water supply engineering Studypool What Are The Components Of Water Answer Key As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of. What Are The Components Of Water Answer Key.
From slidetodoc.com
Amazing Properties of Water Answer Key 1 a What Are The Components Of Water Answer Key Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A compound that prevents sharp,. Identify that water is a compound common to living things. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: This section describes the properties of water in the liquid and. What Are The Components Of Water Answer Key.
From lessonlibraryfrivol.z21.web.core.windows.net
Properties Of Water Biology Notes What Are The Components Of Water Answer Key A compound that prevents sharp,. Because of the higher electronegativity of the oxygen. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. A solution. What Are The Components Of Water Answer Key.
From studylib.net
jjjlm Gizmo Phases of Water Key What Are The Components Of Water Answer Key This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. Because of the higher electronegativity of the oxygen. A compound that prevents sharp,. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Identify that water is. What Are The Components Of Water Answer Key.
From gustavogargiulo.com
2.2 Properties Of Water Answer Key Biology GustavoGargiulo free Scientific Method worksheets What Are The Components Of Water Answer Key As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. A compound that prevents sharp,. Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? A solution with a ph of more than 7. This section. What Are The Components Of Water Answer Key.
From studylib.net
watercycleandanswerkeyworksheet3 What Are The Components Of Water Answer Key This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. Identify that water is a compound common to living things. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Why is water able to. What Are The Components Of Water Answer Key.
From valery-bogspotglover.blogspot.com
A Quick Review of the Properties of Water Answers What Are The Components Of Water Answer Key Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A compound that prevents sharp,. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. A solution with a ph of more than 7. Why is water. What Are The Components Of Water Answer Key.
From db-excel.com
2 2 Properties Of Water Worksheet Answers — What Are The Components Of Water Answer Key Why is water able to form multiple hydrogen bonds, (which account for many of water's special properties)? This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the surface tension and vapor pressure of water. Identify that water is a compound common to living things. Because water seems so ubiquitous, many. What Are The Components Of Water Answer Key.
From studylib.net
Properties of Water Lab What Are The Components Of Water Answer Key Because of the higher electronegativity of the oxygen. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high. A compound that prevents sharp,. This section describes the properties of water in the liquid and solid states and explains how hydrogen bonding affects the. What Are The Components Of Water Answer Key.