What Is Electrochemical Effect Used For . The study of chemical reactions that move electrons is known as electrochemistry. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. Electrochemistry is the study of chemical processes that cause electrons to move.
from schoolbag.info
An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The study of chemical reactions that move electrons is known as electrochemistry. This movement of electrons is called electricity, which can be generated by movements of.
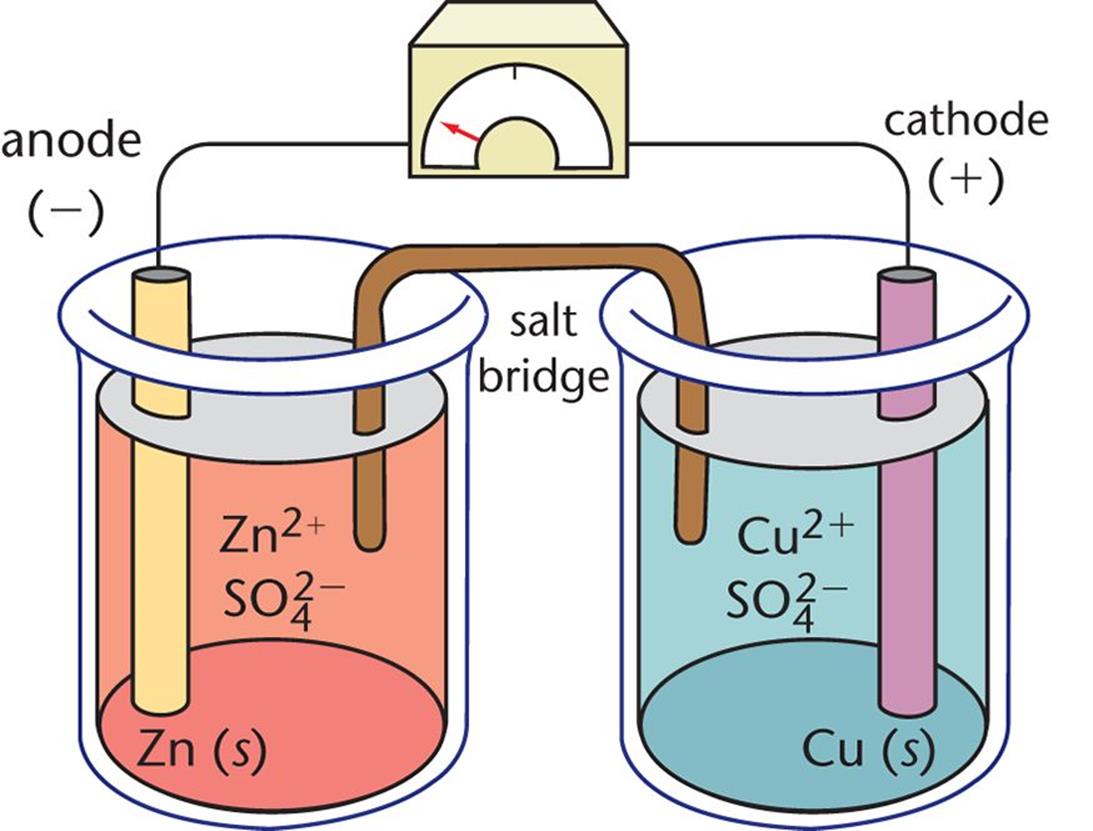
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and copper is the cathode
What Is Electrochemical Effect Used For An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The study of chemical reactions that move electrons is known as electrochemistry. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into.
From mungfali.com
Electrolysis Process Diagram What Is Electrochemical Effect Used For An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. What Is Electrochemical Effect Used For.
From www.pinterest.com
Electrochemistry Electrochemistry, Chemistry lessons, Chemistry experiments What Is Electrochemical Effect Used For The study of chemical reactions that move electrons is known as electrochemistry. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This movement of electrons is called electricity, which can be generated by movements of. As the basic particle of electricity, the electron has an. What Is Electrochemical Effect Used For.
From studylib.net
Electrochemical cells What Is Electrochemical Effect Used For Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The study of chemical reactions. What Is Electrochemical Effect Used For.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses What Is Electrochemical Effect Used For An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The study of chemical reactions that move electrons is known as electrochemistry. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms,. What Is Electrochemical Effect Used For.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation ID2216847 What Is Electrochemical Effect Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemistry is the study of chemical processes that cause electrons to move. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The study of chemical reactions. What Is Electrochemical Effect Used For.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 JEE IIT) YouTube What Is Electrochemical Effect Used For The study of chemical reactions that move electrons is known as electrochemistry. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by. What Is Electrochemical Effect Used For.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 What Is Electrochemical Effect Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. The study of chemical reactions that move electrons is known as electrochemistry. This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry is the study of chemical processes that cause electrons to move. An apparatus that. What Is Electrochemical Effect Used For.
From blog.syrris.com
Electrochemistry made easy with continuous flow chemistry techniques Syrris chemistry blog What Is Electrochemical Effect Used For The study of chemical reactions that move electrons is known as electrochemistry. Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. An apparatus that is used. What Is Electrochemical Effect Used For.
From www.youtube.com
KAC32.7 Electrochemistry Effect of Concentration on Cell Potentials YouTube What Is Electrochemical Effect Used For An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. The study of chemical reactions. What Is Electrochemical Effect Used For.
From www.slideshare.net
Form 4 Chapter 6 Chemistry Electrochemical Series What Is Electrochemical Effect Used For As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely,. What Is Electrochemical Effect Used For.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors What Is Electrochemical Effect Used For An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. The study of chemical reactions that move electrons is known as electrochemistry. Electrochemistry is the study of chemical processes that cause electrons to move. An apparatus that is used. What Is Electrochemical Effect Used For.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu What Is Electrochemical Effect Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemistry is the study of chemical processes that cause electrons to move. The study of chemical reactions that move electrons is known as electrochemistry. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter,. What Is Electrochemical Effect Used For.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts What Is Electrochemical Effect Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any. What Is Electrochemical Effect Used For.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is Electrochemical Effect Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. This movement of electrons is called electricity, which can be generated by movements of. The study of chemical reactions that move electrons is known as electrochemistry. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical. What Is Electrochemical Effect Used For.
From www.researchgate.net
Electrochemical signal transduction. There are four main types of... Download Scientific Diagram What Is Electrochemical Effect Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. As the basic particle of electricity, the electron has an. What Is Electrochemical Effect Used For.
From 2012books.lardbucket.org
Electrochemistry What Is Electrochemical Effect Used For As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry is the study of chemical processes that cause electrons to move. An apparatus that is used to generate electricity. What Is Electrochemical Effect Used For.
From www.researchgate.net
Schematic diagram of an electrochemical water splitting system. Download Scientific Diagram What Is Electrochemical Effect Used For As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This movement of electrons is called electricity, which can be. What Is Electrochemical Effect Used For.
From www.taiwec.com
ELECTROCHEMICAL MACHINING TECHNOLOGY 台電化股份有限公司 What Is Electrochemical Effect Used For As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. The study of chemical reactions that move electrons is known as electrochemistry. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemical cells, also known as. What Is Electrochemical Effect Used For.
From www.researchgate.net
3. Schematic representation of the electrochemical process of... Download Scientific Diagram What Is Electrochemical Effect Used For Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. An apparatus that is used. What Is Electrochemical Effect Used For.
From www.youtube.com
Electrochemical Gradients and Membrane Transport (BIOS 041) YouTube What Is Electrochemical Effect Used For As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The study of chemical reactions that move electrons is known. What Is Electrochemical Effect Used For.
From www.slideserve.com
PPT electrochemistry PowerPoint Presentation ID2683693 What Is Electrochemical Effect Used For Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. The study of chemical reactions that move electrons is known as electrochemistry. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. What Is Electrochemical Effect Used For.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Electrochemical Effect Used For This movement of electrons is called electricity, which can be generated by movements of. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemistry is the study of chemical processes that cause electrons to move. As the basic particle of electricity, the electron has an. What Is Electrochemical Effect Used For.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson What Is Electrochemical Effect Used For As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. Electrochemistry is the study of chemical processes that cause electrons to move. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. What Is Electrochemical Effect Used For.
From loejvnuca.blob.core.windows.net
Electrochemical Reaction Examples at Alice Berke blog What Is Electrochemical Effect Used For This movement of electrons is called electricity, which can be generated by movements of. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the. What Is Electrochemical Effect Used For.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID1837944 What Is Electrochemical Effect Used For An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. As the basic particle of electricity, the electron has an affinity for. What Is Electrochemical Effect Used For.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field What Is Electrochemical Effect Used For Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. An electrochemical cell. What Is Electrochemical Effect Used For.
From www.thoughtco.com
Electrochemical Cell Definition What Is Electrochemical Effect Used For This movement of electrons is called electricity, which can be generated by movements of. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive. What Is Electrochemical Effect Used For.
From pubs.acs.org
On the Temperature Sensitivity of Electrochemical Reaction Thermodynamics ACS Physical What Is Electrochemical Effect Used For The study of chemical reactions that move electrons is known as electrochemistry. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms,. What Is Electrochemical Effect Used For.
From in.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram Cell diagram What Is Electrochemical Effect Used For An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemistry is the study of chemical processes that cause electrons to move. The study of chemical reactions that move electrons is known as electrochemistry. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices. What Is Electrochemical Effect Used For.
From www.researchgate.net
(a) Electrochemical reaction mechanism of the synergistic effect... Download Scientific Diagram What Is Electrochemical Effect Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry. What Is Electrochemical Effect Used For.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and copper is the cathode What Is Electrochemical Effect Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. The study of chemical reactions that move electrons is known as electrochemistry. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. An apparatus that. What Is Electrochemical Effect Used For.
From studylib.net
Electrochemistry What Is Electrochemical Effect Used For As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell is any device that converts. What Is Electrochemical Effect Used For.
From www.researchgate.net
Effect of an alternating potential on an electrochemical cell. Download Scientific Diagram What Is Electrochemical Effect Used For The study of chemical reactions that move electrons is known as electrochemistry. Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. This movement of electrons is called electricity, which can be generated by movements of. An apparatus that is used. What Is Electrochemical Effect Used For.
From saylordotorg.github.io
Electrochemistry What Is Electrochemical Effect Used For This movement of electrons is called electricity, which can be generated by movements of. The study of chemical reactions that move electrons is known as electrochemistry. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell. What Is Electrochemical Effect Used For.
From loejvnuca.blob.core.windows.net
Electrochemical Reaction Examples at Alice Berke blog What Is Electrochemical Effect Used For The study of chemical reactions that move electrons is known as electrochemistry. As the basic particle of electricity, the electron has an affinity for positively charged particles of matter, protons, whether in atoms, groups of atoms, or molecules. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell is any device that converts. What Is Electrochemical Effect Used For.