Sodium Hydroxide Ion Test . A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. Sodium hydroxide solution can be used to identify some metal ions (cations). Therefore, they are identified using flame tests and not by adding sodium. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. Sodium hydroxide is added to the. Ions of group 1 metals (li +, na + and k +) form soluble hydroxides.
from www.slideshare.net
A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. Sodium hydroxide is added to the. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Therefore, they are identified using flame tests and not by adding sodium. Sodium hydroxide solution can be used to identify some metal ions (cations). You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution.
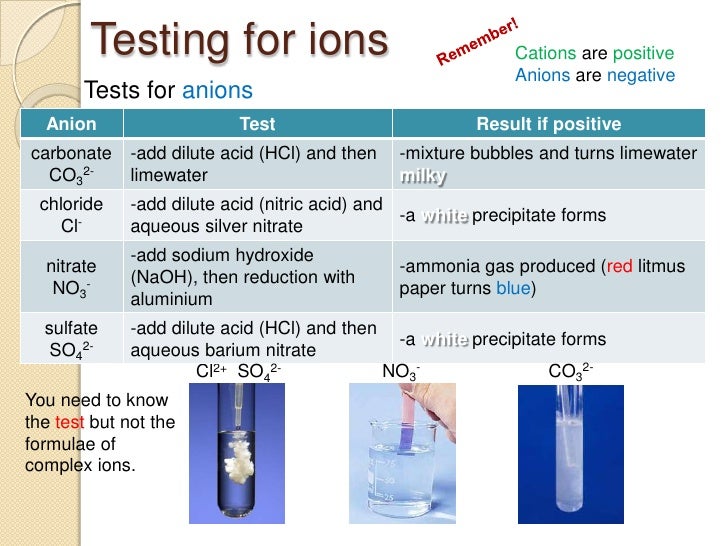
The periodic table and identification of ions
Sodium Hydroxide Ion Test Therefore, they are identified using flame tests and not by adding sodium. The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Sodium hydroxide is added to the. Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. Therefore, they are identified using flame tests and not by adding sodium. Sodium hydroxide solution can be used to identify some metal ions (cations). The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently.
From www.youtube.com
Testing of Aluminium Cations _ sodium hydroxide YouTube Sodium Hydroxide Ion Test You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Sodium hydroxide solution. Sodium Hydroxide Ion Test.
From www.sciencephoto.com
Sodium hydroxide and transition metals Stock Image C028/4198 Sodium Hydroxide Ion Test You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions. Sodium Hydroxide Ion Test.
From www.thesciencehive.co.uk
Identifying Ions (AQA) — the science sauce Sodium Hydroxide Ion Test Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. About 10 drops of. Sodium Hydroxide Ion Test.
From www.youtube.com
AQA Required Practical identifying ions. Sodium hydroxide test for Sodium Hydroxide Ion Test Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. About 10 drops of a solution containing ammonium. Sodium Hydroxide Ion Test.
From www.youtube.com
Test for Cation Test for Ammonium Ion with Aqueous Sodium Hydroxide Sodium Hydroxide Ion Test Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. Sodium hydroxide. Sodium Hydroxide Ion Test.
From www.nagwa.com
Question Video Recalling the Correct Product Formed from the Reaction Sodium Hydroxide Ion Test The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. Therefore, they are identified using flame tests and. Sodium Hydroxide Ion Test.
From www.youtube.com
Testing for Positive Ions with sodium Hydroxide Home learning, KPI Sodium Hydroxide Ion Test Therefore, they are identified using flame tests and not by adding sodium. The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide solution can be used to identify some metal ions (cations). The sodium. Sodium Hydroxide Ion Test.
From www.youtube.com
Lewis Structure of NaOH, sodium hydroxide YouTube Sodium Hydroxide Ion Test Therefore, they are identified using flame tests and not by adding sodium. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. Sodium hydroxide is added to the. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide solution can. Sodium Hydroxide Ion Test.
From www.youtube.com
Practical skills assessment video testing transition metal ions with Sodium Hydroxide Ion Test Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. Therefore, they are identified using flame tests and not by adding sodium. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. You can test for ammonium ions either in a solid or in solution by. Sodium Hydroxide Ion Test.
From www.revisechemistry.uk
Identifying the products of chemical reactions (chemistry only) OCR Sodium Hydroxide Ion Test The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. Sodium hydroxide is added to the. About 10 drops of a solution containing. Sodium Hydroxide Ion Test.
From learnchemistrysaltform4.weebly.com
Confirmatory Test for Cations (Sodium hydroxide as Reagent) Learn Sodium Hydroxide Ion Test Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. The sodium hydroxide test is. Sodium Hydroxide Ion Test.
From www.thesciencehive.co.uk
Chemical tests (GCSE) — the science sauce Sodium Hydroxide Ion Test Sodium hydroxide is added to the. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. Sodium hydroxide solution can be used to identify some metal ions (cations). The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. Therefore, they are identified using flame tests and not by. Sodium Hydroxide Ion Test.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.47 Describe Tests for these Cations NH4 Sodium Hydroxide Ion Test 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. Sodium hydroxide solution can be used to identify some metal ions (cations). Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. Sodium. Sodium Hydroxide Ion Test.
From www.tes.com
Ion Tests Cation Tests Sodium Hydroxide Test Edexcel 91 Separate Sodium Hydroxide Ion Test The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. 7 rows solutions containing copper (ii) ions form a. Sodium Hydroxide Ion Test.
From www.tes.com
Hydroxide Tests GCSE AQA Teaching Resources Sodium Hydroxide Ion Test Sodium hydroxide is added to the. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A few drops of dilute sodium hydroxide. Sodium Hydroxide Ion Test.
From chemistry.com.pk
Testing for Cations By Sodium Hydroxide & Ammonia Precipitates Sodium Hydroxide Ion Test 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. Sodium hydroxide is added to the. Sodium hydroxide solution can be used to identify some metal ions (cations). About 10 drops of. Sodium Hydroxide Ion Test.
From www.savemyexams.com
Testing for Halide Ions AQA A Level Chemistry Revision Notes 2017 Sodium Hydroxide Ion Test A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. Sodium hydroxide solution can be used. Sodium Hydroxide Ion Test.
From www.youtube.com
Cation Test Zinc ion with Sodium Hydroxide YouTube Sodium Hydroxide Ion Test You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. Therefore, they are identified using flame tests and not by adding sodium. About 10 drops of a solution containing. Sodium Hydroxide Ion Test.
From www.thesciencehive.co.uk
Chemical tests (GCSE) — the science hive Sodium Hydroxide Ion Test A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. About 10 drops. Sodium Hydroxide Ion Test.
From www.hanlin.com
AQA A Level Chemistry复习笔记4.2.2 Identifying Anions & Cations翰林国际教育 Sodium Hydroxide Ion Test Sodium hydroxide solution can be used to identify some metal ions (cations). You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Solutions of aluminium, calcium and magnesium ions form. Sodium Hydroxide Ion Test.
From www.youtube.com
GCSE CHEMISTRY CHEMICAL TESTS LESSON 2 test for cations aqueous Sodium Hydroxide Ion Test A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. Therefore, they are identified using flame tests and not by adding sodium. Sodium hydroxide is added to. Sodium Hydroxide Ion Test.
From www.scribd.com
Tests for Ions and Gases Sodium Hydroxide Sodium Hydroxide Ion Test Sodium hydroxide solution can be used to identify some metal ions (cations). Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. Ions of group 1 metals (li +, na + and k +) form. Sodium Hydroxide Ion Test.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Sodium Hydroxide Ion Test Sodium hydroxide is added to the. Therefore, they are identified using flame tests and not by adding sodium. Ions of group 1 metals (li +, na + and k +) form soluble hydroxides. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. 7 rows solutions containing copper (ii). Sodium Hydroxide Ion Test.
From www.youtube.com
QA Test for Iron(III) Ion with aqueous sodium hydroxide YouTube Sodium Hydroxide Ion Test About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Sodium hydroxide solution can be used to identify some metal ions (cations). Sodium hydroxide is added to the. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. You can test for ammonium ions either in. Sodium Hydroxide Ion Test.
From www.youtube.com
Testing for Cations Using Sodium Hydroxide solution YouTube Sodium Hydroxide Ion Test You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. Sodium hydroxide solution can be used to identify some metal ions (cations). The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. A few drops of dilute sodium hydroxide solution react to. Sodium Hydroxide Ion Test.
From socratic.org
Sodium hydroxide (NaOH) is classified as a strong base. For every mole Sodium Hydroxide Ion Test Sodium hydroxide is added to the. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Therefore, they are identified using flame tests and not by adding sodium. Ions of group 1 metals. Sodium Hydroxide Ion Test.
From www.youtube.com
QA Lab Practical Test for Cations Copper(II) ion, Cu2+ with aqueous Sodium Hydroxide Ion Test Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. Sodium hydroxide is added to the. Therefore, they are identified using flame tests and not by adding sodium. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide solution can be used to identify some metal. Sodium Hydroxide Ion Test.
From www.onlinechemistrytutor.net
metalhydroxidetestscopy001 Online Chemistry Tutor Sodium Hydroxide Ion Test The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. Sodium hydroxide is added to the. Sodium hydroxide. Sodium Hydroxide Ion Test.
From www.youtube.com
Identifying Positive Ions (Test by NaOH and NH3) Acid, Base and Salt Sodium Hydroxide Ion Test The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. Sodium hydroxide solution can be used to identify some metal ions (cations). You can test for ammonium ions either in a solid or in. Sodium Hydroxide Ion Test.
From www.youtube.com
Test for Copper (II) ion with Sodium Hydroxide YouTube Sodium Hydroxide Ion Test Sodium hydroxide is added to the. You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. Therefore, they are identified using flame tests and not by adding sodium. The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. About 10 drops of. Sodium Hydroxide Ion Test.
From www.youtube.com
Test for Cations NaOH YouTube Sodium Hydroxide Ion Test The sodium hydroxide test is a qualitative chemical analysis method used to identify metallic cations in solutions. Sodium hydroxide solution can be used to identify some metal ions (cations). You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. The sodium hydroxide test is a chemical test used to. Sodium Hydroxide Ion Test.
From www.youtube.com
Test for ammonium ions/cations using sodium hydroxide NaOH GCSE YouTube Sodium Hydroxide Ion Test Sodium hydroxide solution can be used to identify some metal ions (cations). About 10 drops of a solution containing ammonium ions, such as ammonium chloride, should be added to a clean. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Ions of group 1 metals (li +, na + and k +). Sodium Hydroxide Ion Test.
From www.slideshare.net
The periodic table and identification of ions Sodium Hydroxide Ion Test You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. Sodium hydroxide solution can be used to identify some metal ions (cations). The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. A few drops of dilute sodium hydroxide. Sodium Hydroxide Ion Test.
From www.hobbyhomebrew.com
Sodium Hydroxide 4 oz Reagent for BSG Acid Test Kit Certified 0.1 Sodium Hydroxide Ion Test You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is. The sodium hydroxide test is a chemical test used to identify the presence of certain metal ions in a solution. Sodium hydroxide is added. Sodium Hydroxide Ion Test.
From www.youtube.com
How is the concentration of hydroxide ions affected when excess base Sodium Hydroxide Ion Test You can test for ammonium ions either in a solid or in solution by adding sodium hydroxide solution and warming gently. Sodium hydroxide is added to the. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide solution can be used to identify some metal ions (cations). Ions of group 1. Sodium Hydroxide Ion Test.