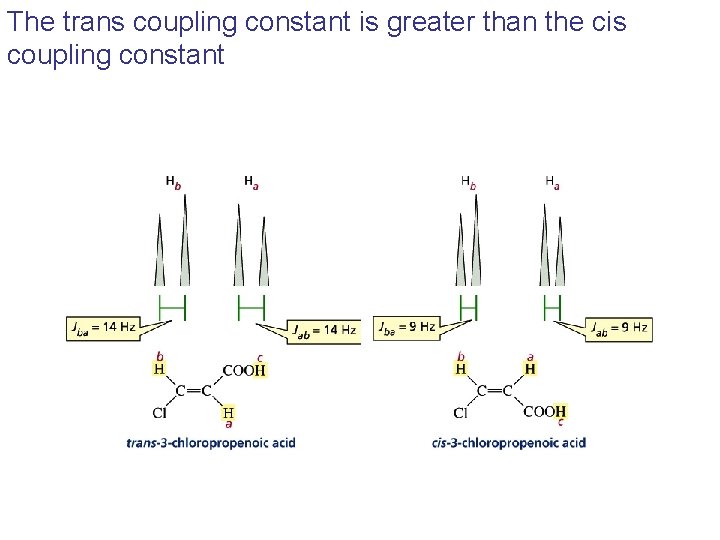
Cis Coupling Constant Nmr . Here are a couple of terms to know: • the general form of the karplus relationship is: Which one of these spectra corresponds to that of ethanol?. 3j(φ) = a cos2(φ) + b cos(φ) + c. Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. A simple and efficient approach for stereochemical. Cis and trans coupling appear differently on 1 h nmr spectrum. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Coupling constants chem 117 review: Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings.
from slidetodoc.com
Which one of these spectra corresponds to that of ethanol?. Coupling constants chem 117 review: 3j(φ) = a cos2(φ) + b cos(φ) + c. Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Here are a couple of terms to know: Cis and trans coupling appear differently on 1 h nmr spectrum. A simple and efficient approach for stereochemical. • the general form of the karplus relationship is:
Chapter 14 NMR Spectroscopy Organic Chemistry 4 th
Cis Coupling Constant Nmr The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Cis and trans coupling appear differently on 1 h nmr spectrum. Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. Here are a couple of terms to know: • the general form of the karplus relationship is: Which one of these spectra corresponds to that of ethanol?. Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. 3j(φ) = a cos2(φ) + b cos(φ) + c. A simple and efficient approach for stereochemical. Coupling constants chem 117 review: The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms.
From www.youtube.com
NMR Spectroscopy Coupling Constants YouTube Cis Coupling Constant Nmr Which one of these spectra corresponds to that of ethanol?. • the general form of the karplus relationship is: Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. A simple and efficient approach for stereochemical. Here are a couple of terms to know: 3j(φ). Cis Coupling Constant Nmr.
From www.researchgate.net
1 H NMR 3 J coupling constants (red arrows, φ ≈ 180°; blue arrows, φ ≈ Cis Coupling Constant Nmr The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. Here are a couple of terms to know: 3j(φ) =. Cis Coupling Constant Nmr.
From slidetodoc.com
Chapter 14 NMR Spectroscopy Organic Chemistry 4 th Cis Coupling Constant Nmr Which one of these spectra corresponds to that of ethanol?. Cis and trans coupling appear differently on 1 h nmr spectrum. Here are a couple of terms to know: Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity. Cis Coupling Constant Nmr.
From www.researchgate.net
NMR spinspin coupling constants [Hz] for HF, HXeF, (HF) 2 and HXeFÁ Á Cis Coupling Constant Nmr Coupling constants chem 117 review: Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. 3j(φ) = a cos2(φ) + b cos(φ) + c. Here are a couple of terms to know: The coupling constant, denoted as j, represents the splitting of nmr signals observed. Cis Coupling Constant Nmr.
From giowgjjlu.blob.core.windows.net
Coupling Constant In 13C Nmr at Mitchell Hubbard blog Cis Coupling Constant Nmr 3j(φ) = a cos2(φ) + b cos(φ) + c. • the general form of the karplus relationship is: Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. Cis and trans coupling appear differently on 1 h nmr spectrum. Coupling constants chem 117 review: Determining. Cis Coupling Constant Nmr.
From www.studypool.com
SOLUTION NMR spectroscopy coupling constant Studypool Cis Coupling Constant Nmr Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. Coupling constants chem 117 review: 3j(φ) = a cos2(φ) + b cos(φ) + c. Cis and trans coupling appear differently on 1 h nmr spectrum. Which one of these spectra corresponds to that of ethanol?.. Cis Coupling Constant Nmr.
From mavink.com
Coupling Constant In Nmr Spectroscopy Cis Coupling Constant Nmr Here are a couple of terms to know: • the general form of the karplus relationship is: 3j(φ) = a cos2(φ) + b cos(φ) + c. Which one of these spectra corresponds to that of ethanol?. A simple and efficient approach for stereochemical. Cis and trans coupling appear differently on 1 h nmr spectrum. Determining the signs and magnitudes of. Cis Coupling Constant Nmr.
From www.slideserve.com
PPT Coupling Constants (J) PowerPoint Presentation ID3561615 Cis Coupling Constant Nmr Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. Which one of these spectra corresponds to that of ethanol?. The coupling constant, denoted as. Cis Coupling Constant Nmr.
From www.slideserve.com
PPT Coupling Constants PowerPoint Presentation, free download ID Cis Coupling Constant Nmr A simple and efficient approach for stereochemical. Which one of these spectra corresponds to that of ethanol?. • the general form of the karplus relationship is: Cis and trans coupling appear differently on 1 h nmr spectrum. Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d. Cis Coupling Constant Nmr.
From www.studypool.com
SOLUTION Factors affecting the coupling constant (proton NMR) Studypool Cis Coupling Constant Nmr • the general form of the karplus relationship is: Which one of these spectra corresponds to that of ethanol?. Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh. Cis Coupling Constant Nmr.
From www.youtube.com
Coupling constant in protonNMR continued (Part 9) YouTube Cis Coupling Constant Nmr Coupling constants chem 117 review: 3j(φ) = a cos2(φ) + b cos(φ) + c. Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. A simple and efficient approach for stereochemical. Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific. Cis Coupling Constant Nmr.
From giowgjjlu.blob.core.windows.net
Coupling Constant In 13C Nmr at Mitchell Hubbard blog Cis Coupling Constant Nmr A simple and efficient approach for stereochemical. Which one of these spectra corresponds to that of ethanol?. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a. Cis Coupling Constant Nmr.
From mavink.com
Coupling Constant In Nmr Spectroscopy Cis Coupling Constant Nmr Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. 3j(φ) = a cos2(φ) + b cos(φ) + c. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Determining. Cis Coupling Constant Nmr.
From chempedia.info
NMR. Coupling constants Big Chemical Encyclopedia Cis Coupling Constant Nmr 3j(φ) = a cos2(φ) + b cos(φ) + c. Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. A simple and efficient approach for. Cis Coupling Constant Nmr.
From www.researchgate.net
What kind of NMR coupling this might be? Cis Coupling Constant Nmr Here are a couple of terms to know: Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. A simple and efficient approach for stereochemical.. Cis Coupling Constant Nmr.
From www.numerade.com
SOLVED NMR Correlation Table Coupling Constants Type of proton RCH R Cis Coupling Constant Nmr Which one of these spectra corresponds to that of ethanol?. Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. • the general form of. Cis Coupling Constant Nmr.
From www.scribd.com
NMR Coupling Nuclear Resonance Spectroscopy Nuclear Cis Coupling Constant Nmr 3j(φ) = a cos2(φ) + b cos(φ) + c. A simple and efficient approach for stereochemical. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Which one of these spectra corresponds to that of ethanol?. Determining the signs and magnitudes of j hf couplings. Cis Coupling Constant Nmr.
From www.studypool.com
SOLUTION Detail about coupling constant proton nmr spectroscopy msc Cis Coupling Constant Nmr Which one of these spectra corresponds to that of ethanol?. Cis and trans coupling appear differently on 1 h nmr spectrum. Here are a couple of terms to know: Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. • the general form of the. Cis Coupling Constant Nmr.
From mavink.com
Coupling Constant In Nmr Spectroscopy Cis Coupling Constant Nmr Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a. Cis Coupling Constant Nmr.
From chempedia.info
1H NMR coupling constants Big Chemical Encyclopedia Cis Coupling Constant Nmr Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a. Cis Coupling Constant Nmr.
From klafhmunm.blob.core.windows.net
Coupling Constant Explanation at Julie Hunt blog Cis Coupling Constant Nmr • the general form of the karplus relationship is: Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. Cis and trans coupling appear differently on 1 h nmr spectrum. Coupling constants chem 117 review: Determining the signs and magnitudes of j hf couplings is. Cis Coupling Constant Nmr.
From u-of-o-nmr-facility.blogspot.com
University of Ottawa NMR Facility Blog E.COSY and the Relative Signs Cis Coupling Constant Nmr Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. • the general form of the karplus relationship is: Here are a couple of terms. Cis Coupling Constant Nmr.
From klaoprxuq.blob.core.windows.net
Coupling Constant Cis Trans Alkene at Sydney Gill blog Cis Coupling Constant Nmr Here are a couple of terms to know: 3j(φ) = a cos2(φ) + b cos(φ) + c. Cis and trans coupling appear differently on 1 h nmr spectrum. Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. A simple and efficient approach for stereochemical.. Cis Coupling Constant Nmr.
From www.slideserve.com
PPT Interpreting 1 H (Proton) NMR Spectra PowerPoint Presentation Cis Coupling Constant Nmr Cis and trans coupling appear differently on 1 h nmr spectrum. A simple and efficient approach for stereochemical. 3j(φ) = a cos2(φ) + b cos(φ) + c. Which one of these spectra corresponds to that of ethanol?. Coupling constants chem 117 review: The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to. Cis Coupling Constant Nmr.
From chem.libretexts.org
Typical coupling constants in NMR Chemistry LibreTexts Cis Coupling Constant Nmr Here are a couple of terms to know: Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific structural information, but is often difficult in conventional 1d 1 h nmr because of the added complexity of multiplet structure that is caused by both j hh and j hf couplings. Which one of these spectra corresponds to. Cis Coupling Constant Nmr.
From www.youtube.com
Coupling constant in protonNMR continued (Part 8) YouTube Cis Coupling Constant Nmr Here are a couple of terms to know: The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Coupling constants chem 117 review: Cis and trans coupling appear differently on 1 h nmr spectrum. A simple and efficient approach for stereochemical. • the general form. Cis Coupling Constant Nmr.
From giowgjjlu.blob.core.windows.net
Coupling Constant In 13C Nmr at Mitchell Hubbard blog Cis Coupling Constant Nmr 3j(φ) = a cos2(φ) + b cos(φ) + c. • the general form of the karplus relationship is: Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. Cis and trans coupling appear differently on 1 h nmr spectrum. The coupling constant, denoted as j,. Cis Coupling Constant Nmr.
From loeagychc.blob.core.windows.net
Coupling Constant Formula Physics at Gerald Phillips blog Cis Coupling Constant Nmr Cis and trans coupling appear differently on 1 h nmr spectrum. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Which one of these spectra corresponds to that of ethanol?. Determining the signs and magnitudes of j hf couplings is crucial for obtaining stereospecific. Cis Coupling Constant Nmr.
From www.youtube.com
CSIR NET Dec 2023 Chemistry Solution Coupling constant for cis and Cis Coupling Constant Nmr Here are a couple of terms to know: Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. A simple and efficient approach for stereochemical. Coupling constants chem 117 review: Which one of these spectra corresponds to that of ethanol?. The coupling constant, denoted as. Cis Coupling Constant Nmr.
From giowgjjlu.blob.core.windows.net
Coupling Constant In 13C Nmr at Mitchell Hubbard blog Cis Coupling Constant Nmr Coupling constants chem 117 review: Cis and trans coupling appear differently on 1 h nmr spectrum. Which one of these spectra corresponds to that of ethanol?. A simple and efficient approach for stereochemical. Here are a couple of terms to know: • the general form of the karplus relationship is: Determining the signs and magnitudes of j hf couplings is. Cis Coupling Constant Nmr.
From www.researchgate.net
The selected 13 C NMR chemical shift (d in ppm) and coupling constants Cis Coupling Constant Nmr The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. • the general form of the karplus relationship is: Which one of these spectra corresponds to that of ethanol?. 3j(φ) = a cos2(φ) + b cos(φ) + c. Maximum coupling constant (j) occurs at 0˚. Cis Coupling Constant Nmr.
From www.youtube.com
Coupling constant in protonNMR (Part 7) YouTube Cis Coupling Constant Nmr 3j(φ) = a cos2(φ) + b cos(φ) + c. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Here are a couple of terms to know: Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a. Cis Coupling Constant Nmr.
From hatsudy.com
NMR Coupling of Benzene Rings OrthoMeta Peak and Chemical Shifts Cis Coupling Constant Nmr Maximum coupling constant (j) occurs at 0˚ and 180˚ (eclipsed and anti protons, respectively), and is at a minimum when they are at 90˚ (orthogonal. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. Which one of these spectra corresponds to that of ethanol?.. Cis Coupling Constant Nmr.
From www.researchgate.net
1 H NMR onedimensional spectra of double bond region of... Download Cis Coupling Constant Nmr • the general form of the karplus relationship is: Cis and trans coupling appear differently on 1 h nmr spectrum. Which one of these spectra corresponds to that of ethanol?. Coupling constants chem 117 review: A simple and efficient approach for stereochemical. Here are a couple of terms to know: Determining the signs and magnitudes of j hf couplings is. Cis Coupling Constant Nmr.
From xyrzkdiuum.blogspot.com
How To Calculate Coupling Constant For Doublet Of Doublet Higher peak Cis Coupling Constant Nmr A simple and efficient approach for stereochemical. The coupling constant, denoted as j, represents the splitting of nmr signals observed in a spectrum due to the magnetic interactions between neighboring hydrogen atoms. 3j(φ) = a cos2(φ) + b cos(φ) + c. • the general form of the karplus relationship is: Cis and trans coupling appear differently on 1 h nmr. Cis Coupling Constant Nmr.