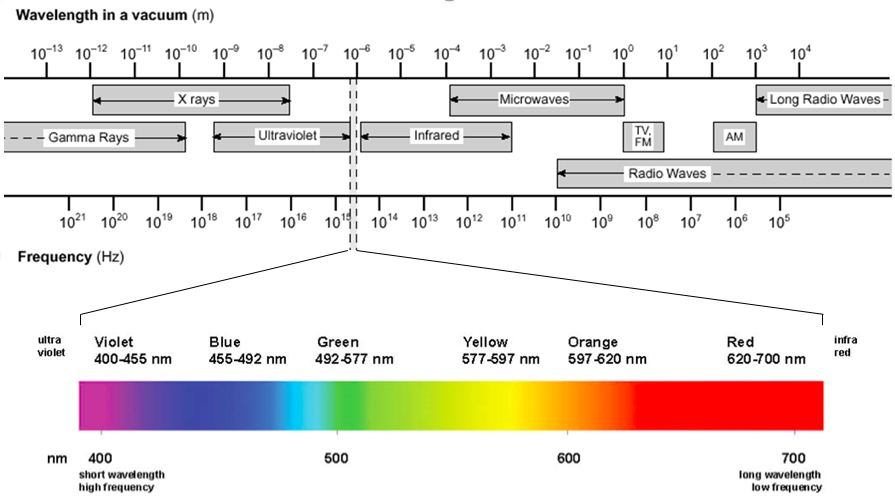
Light And Spectroscopy . Chemistry archive > unit 1. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Bohr's model of the hydrogen atom. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Some properties of electromagnetic radiation, such as its refraction. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. By performing this dissection and. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Interaction of light and matter.
from chem.libretexts.org
By performing this dissection and. Chemistry archive > unit 1. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Interaction of light and matter. Some properties of electromagnetic radiation, such as its refraction. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Bohr's model of the hydrogen atom. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies).
10 Introduction to Spectroscopy Chemistry LibreTexts
Light And Spectroscopy Chemistry archive > unit 1. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Interaction of light and matter. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Some properties of electromagnetic radiation, such as its refraction. Chemistry archive > unit 1. By performing this dissection and. Bohr's model of the hydrogen atom. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies).
From www.wisegeek.com
What is a Spectroscope? (with pictures) Light And Spectroscopy Some properties of electromagnetic radiation, such as its refraction. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Bohr's model of the hydrogen. Light And Spectroscopy.
From chem.libretexts.org
10 Introduction to Spectroscopy Chemistry LibreTexts Light And Spectroscopy Bohr's model of the hydrogen atom. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). By performing this dissection and. Some properties of electromagnetic radiation, such as its refraction. In spectroscopy, we use light to determine. Light And Spectroscopy.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Light And Spectroscopy Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. By performing this dissection and. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Some properties of electromagnetic radiation, such as its refraction. Interaction of light. Light And Spectroscopy.
From www.en.silicann.com
Spectrometers How they work and what they are for Light And Spectroscopy In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength. Light And Spectroscopy.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Light And Spectroscopy Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Bohr's model of the hydrogen atom. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Interaction of light and matter. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Spectroscopy,. Light And Spectroscopy.
From blog.sdss.org
How SDSS Uses Light to Measure the Mass of Stars in Galaxies Science Light And Spectroscopy Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Interaction of light and matter. Chemistry archive > unit 1. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Bohr's model of the hydrogen atom. Spectroscopy. Light And Spectroscopy.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Light And Spectroscopy Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Interaction of light and matter. Chemistry archive > unit 1. Bohr's model of the hydrogen atom. Spectroscopy, study of the absorption and emission of light and other radiation by matter,. Light And Spectroscopy.
From www.researchgate.net
Emission spectra of different light sources (a) incandescent tungsten Light And Spectroscopy Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Bohr's model of the hydrogen atom. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Some properties of electromagnetic radiation, such. Light And Spectroscopy.
From mybiologydictionary.com
Spectroscopy Definition and its Types Light And Spectroscopy In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Chemistry archive > unit 1. Electromagnetic radiation—light—is a form of. Light And Spectroscopy.
From psiberg.com
UVVis Spectroscopy Principle, Instrumentation, and Applications Light And Spectroscopy Chemistry archive > unit 1. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). In spectroscopy, we use light to determine. Light And Spectroscopy.
From www.thoughtco.com
Spectroscopy Introduction Light And Spectroscopy Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Some properties of electromagnetic radiation, such as its refraction. Bohr's model of the hydrogen atom. Interaction of light and matter. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). By performing this dissection and. Light has. Light And Spectroscopy.
From www.thermofisher.cn
The Light Spectrum and Its Relationship With Fluorescence Thermo Light And Spectroscopy By performing this dissection and. Interaction of light and matter. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Some properties of electromagnetic radiation, such as its refraction. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of. Light And Spectroscopy.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Light And Spectroscopy Bohr's model of the hydrogen atom. Some properties of electromagnetic radiation, such as its refraction. Chemistry archive > unit 1. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Interaction of light and matter. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as. Light And Spectroscopy.
From spiff.rit.edu
Spectrographs and Spectra Light And Spectroscopy Chemistry archive > unit 1. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Bohr's model. Light And Spectroscopy.
From www.astronoo.com
Spectroscopy — Astronoo Light And Spectroscopy Bohr's model of the hydrogen atom. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Chemistry archive > unit 1. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Interaction of light and matter. In. Light And Spectroscopy.
From exoplanets.nasa.gov
Spectroscopy Detection of Biosignatures Exploration Light And Spectroscopy Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Interaction of light and matter. Bohr's model of the hydrogen atom. By. Light And Spectroscopy.
From www.youtube.com
Light and Spectroscopy Lesson YouTube Light And Spectroscopy Some properties of electromagnetic radiation, such as its refraction. Bohr's model of the hydrogen atom. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Spectroscopy pertains to the dispersion of an. Light And Spectroscopy.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Light And Spectroscopy Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Some properties of electromagnetic radiation, such as. Light And Spectroscopy.
From study.com
Spectroscope Definition, Diagram & Parts Lesson Light And Spectroscopy Chemistry archive > unit 1. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. By performing this dissection and. Some properties of electromagnetic radiation, such as. Light And Spectroscopy.
From webbtelescope.org
Spectroscopy 101 Introduction b Light And Spectroscopy Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Interaction of light and matter. Some properties of electromagnetic radiation, such as its refraction. Bohr's model of. Light And Spectroscopy.
From ruby-sapphire.com
Introduction to Infrared Spectroscopy (FTIR) in Gemology Light And Spectroscopy Chemistry archive > unit 1. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Light has a number of fascinating (and somewhat odd) characteristics that are. Light And Spectroscopy.
From depositphotos.com
Spectroscopy vector illustration. Matter and radiation Light And Spectroscopy Interaction of light and matter. Some properties of electromagnetic radiation, such as its refraction. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles.. Light And Spectroscopy.
From lightandsoundinmoderntec.weebly.com
Spectroscopy Science Project Light And Spectroscopy Interaction of light and matter. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. By performing this dissection and. Some properties of electromagnetic radiation, such as its refraction. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties. Light And Spectroscopy.
From pixels.com
Prism Dispersing Light Into Spectrum Photograph by David Parker Pixels Light And Spectroscopy Chemistry archive > unit 1. Interaction of light and matter. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Bohr's model of the hydrogen atom. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Light. Light And Spectroscopy.
From www.en.silicann.com
The difference between spectroscope, spectrometer and spectrophotometer Light And Spectroscopy Chemistry archive > unit 1. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Electromagnetic radiation—light—is a form of energy whose behavior is described by the. Light And Spectroscopy.
From atascientific.com.au
Understanding Spectrometry and Spectroscopy ATA Scientific Light And Spectroscopy In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Bohr's model of the hydrogen atom. Chemistry archive > unit 1. Light. Light And Spectroscopy.
From exodtihxe.blob.core.windows.net
What Does A Spectrograph Do To Light That Enters It at Ella Byrd blog Light And Spectroscopy Some properties of electromagnetic radiation, such as its refraction. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. Light And Spectroscopy.
From microbiologynotes.org
uv vis spectroscopy Microbiology Notes Light And Spectroscopy In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Interaction of light and matter. By performing this dissection and. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy.. Light And Spectroscopy.
From webbtelescope.org
Spectroscopy 101 Light and Matter b Light And Spectroscopy Some properties of electromagnetic radiation, such as its refraction. Interaction of light and matter. Chemistry archive > unit 1. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). By performing this dissection and. In spectroscopy, we. Light And Spectroscopy.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Light And Spectroscopy Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Interaction of light and matter. Bohr's model of the hydrogen atom. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Spectroscopy, study of the absorption and emission of light and other radiation by matter,. Light And Spectroscopy.
From www.smacgigworld.com
UVVis Spectroscopy Principle Light And Spectroscopy In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. By performing this dissection and. Bohr's model of the hydrogen atom. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Some. Light And Spectroscopy.
From medium.com
Light and Dark Understanding Spectrometry by Amelia Settembre Medium Light And Spectroscopy Chemistry archive > unit 1. Bohr's model of the hydrogen atom. Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both waves and particles. Spectroscopy pertains to. Light And Spectroscopy.
From www.sunderlandastro.com
16th December Lecture Spectroscopy for Beginners Sunderland Light And Spectroscopy Some properties of electromagnetic radiation, such as its refraction. Bohr's model of the hydrogen atom. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Electromagnetic radiation—light—is a form of energy whose behavior is described. Light And Spectroscopy.
From webbtelescope.org
Spectroscopy 101 Light and Matter b Light And Spectroscopy By performing this dissection and. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and electron and. Interaction of light and matter. Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Electromagnetic radiation—light—is a form of energy whose behavior is described by the properties of both. Light And Spectroscopy.
From users.highland.edu
Atomic Spectra and Models of the Atom Light And Spectroscopy Bohr's model of the hydrogen atom. Interaction of light and matter. Spectroscopy pertains to the dispersion of an object’s light into its component colors (i.e., energies). Light has a number of fascinating (and somewhat odd) characteristics that are important for spectroscopy. Some properties of electromagnetic radiation, such as its refraction. Spectroscopy, study of the absorption and emission of light and. Light And Spectroscopy.