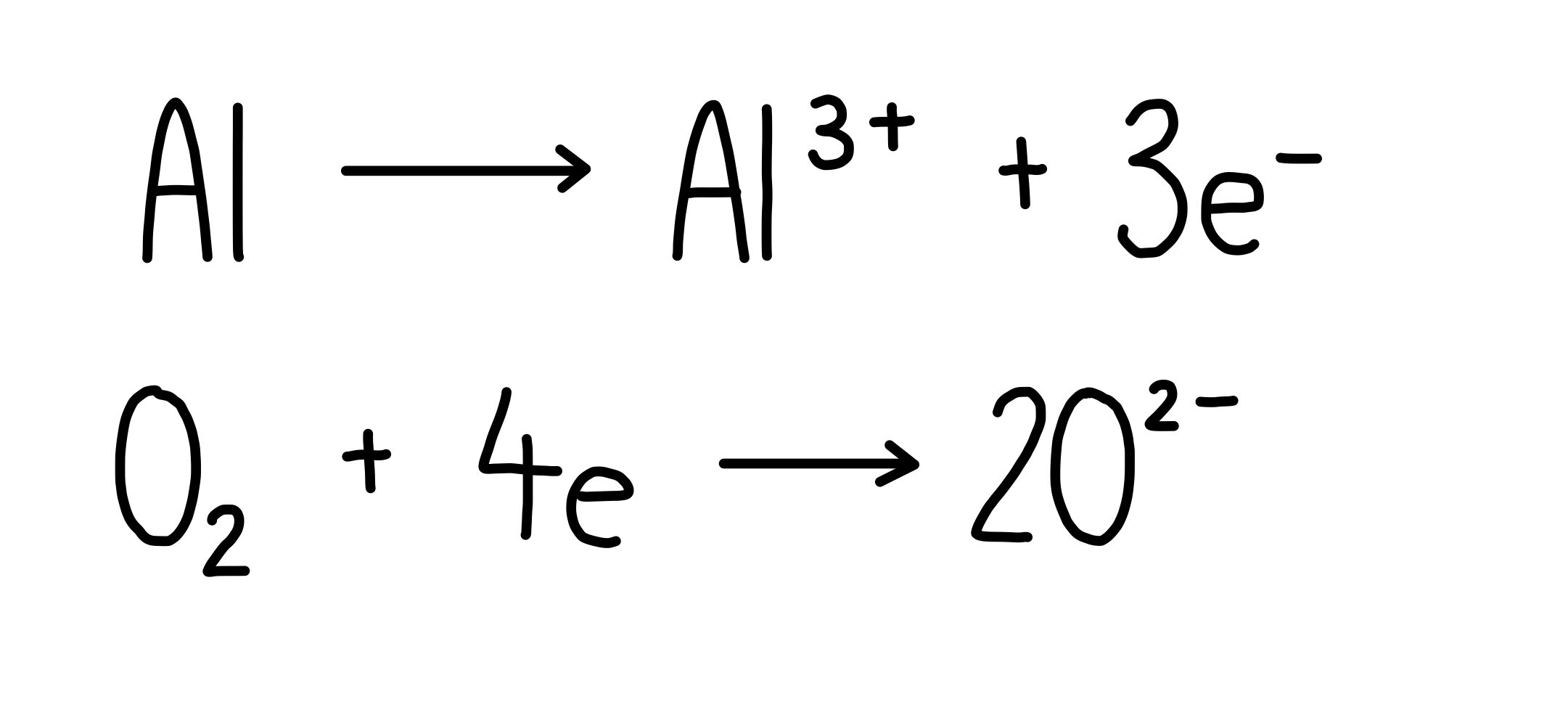What Is The Balanced Equation For Aluminum And Oxygen . The balanced chemical equation is: Al + o2 → al2o3. 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. We're given that aluminum (al) can react with oxygen gas (o2; The balanced equation will appear. The balanced chemical equation for this. The balanced equation for aluminum and oxygen is: Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. Achieve a common 6 oxygen atoms on either side of the. In a reaction between aluminium and. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). This is the unbalanced equation described. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This balanced equation ensures that the number of atoms of each.
from www.thesciencehive.co.uk
To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). Al + o2 → al2o3. This balanced equation ensures that the number of atoms of each. The balanced chemical equation is: We're given that aluminum (al) can react with oxygen gas (o2; The balanced equation will appear. 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. In a reaction between aluminium and. Achieve a common 6 oxygen atoms on either side of the.
Redox and Electrode Potentials — the science hive
What Is The Balanced Equation For Aluminum And Oxygen The balanced equation for aluminum and oxygen is: In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). Al + o2 → al2o3. We're given that aluminum (al) can react with oxygen gas (o2; Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. Achieve a common 6 oxygen atoms on either side of the. The balanced chemical equation is: The balanced chemical equation for this. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This is the unbalanced equation described. In a reaction between aluminium and. This balanced equation ensures that the number of atoms of each. The balanced equation for aluminum and oxygen is: 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. The balanced equation will appear.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas What Is The Balanced Equation For Aluminum And Oxygen In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. The balanced chemical equation is: In a reaction between aluminium and. The balanced chemical equation for this. The balanced equation for aluminum and oxygen is: Achieve a common 6 oxygen atoms on either side of the. 4al(s) +3o2(g) → 2al2o3(s) according to the. What Is The Balanced Equation For Aluminum And Oxygen.
From www.numerade.com
SOLVEDAluminum oxidizes to form aluminum oxide. a. Write the balanced What Is The Balanced Equation For Aluminum And Oxygen The balanced equation will appear. Achieve a common 6 oxygen atoms on either side of the. We're given that aluminum (al) can react with oxygen gas (o2; Al + o2 → al2o3. This balanced equation ensures that the number of atoms of each. The balanced chemical equation for this. The balanced equation for aluminum and oxygen is: In a reaction. What Is The Balanced Equation For Aluminum And Oxygen.
From www.slideserve.com
PPT Reactions and Equations PowerPoint Presentation, free download What Is The Balanced Equation For Aluminum And Oxygen In a reaction between aluminium and. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). Achieve a common 6 oxygen atoms on either side of the. The balanced equation will appear. The balanced chemical equation for this. Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. The balanced chemical equation. What Is The Balanced Equation For Aluminum And Oxygen.
From www.meritnation.com
Write balanced chemical equation for Aluminium oxide and sodium oxide What Is The Balanced Equation For Aluminum And Oxygen Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. This balanced equation ensures that the number of atoms of each. In this problem, we. What Is The Balanced Equation For Aluminum And Oxygen.
From readthescience.blogspot.com
READ THE SCIENCE 10.1 The reaction of metals with oxygen What Is The Balanced Equation For Aluminum And Oxygen The balanced equation will appear. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Al + o2 → al2o3. The balanced chemical equation for this. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In a reaction between aluminium and. Don't forget it's diatomic!). What Is The Balanced Equation For Aluminum And Oxygen.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free What Is The Balanced Equation For Aluminum And Oxygen This is the unbalanced equation described. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). The balanced chemical equation is: The balanced equation will appear. Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles. What Is The Balanced Equation For Aluminum And Oxygen.
From www.numerade.com
SOLVEDThe balanced equation for the reaction of aluminum metal and What Is The Balanced Equation For Aluminum And Oxygen The balanced chemical equation for this. 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. The balanced equation will appear. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). Achieve a common 6 oxygen atoms. What Is The Balanced Equation For Aluminum And Oxygen.
From www.slideserve.com
PPT Unit 8 Chemical Reactions PowerPoint Presentation, free download What Is The Balanced Equation For Aluminum And Oxygen We're given that aluminum (al) can react with oxygen gas (o2; In a reaction between aluminium and. The balanced chemical equation for this. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). The balanced equation for aluminum and oxygen is: 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. Al + o2. What Is The Balanced Equation For Aluminum And Oxygen.
From brainly.in
3. Write balanced chemical equation.When Aluminium reacts with oxygen What Is The Balanced Equation For Aluminum And Oxygen The balanced chemical equation is: This is the unbalanced equation described. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. This balanced equation ensures that the number of atoms of each. 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. The balanced equation will. What Is The Balanced Equation For Aluminum And Oxygen.
From slideplayer.com
Balancing Chemical Equations ppt download What Is The Balanced Equation For Aluminum And Oxygen In a reaction between aluminium and. 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. The balanced chemical equation is: Al + o2 → al2o3. This is the unbalanced equation described. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Symbol for aluminium is. What Is The Balanced Equation For Aluminum And Oxygen.
From www.museoinclusivo.com
Exploring the Formulas of Aluminum and Oxygen Bonding, Balancing, and What Is The Balanced Equation For Aluminum And Oxygen Achieve a common 6 oxygen atoms on either side of the. The balanced chemical equation is: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). We're given that aluminum (al) can react with oxygen gas (o2; 4al(s) +3o2(g). What Is The Balanced Equation For Aluminum And Oxygen.
From www.slideserve.com
PPT What is the difference between a chemical reaction and physical What Is The Balanced Equation For Aluminum And Oxygen Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. The balanced chemical equation is: 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). The balanced equation will appear. This is the unbalanced equation. What Is The Balanced Equation For Aluminum And Oxygen.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas What Is The Balanced Equation For Aluminum And Oxygen This balanced equation ensures that the number of atoms of each. We're given that aluminum (al) can react with oxygen gas (o2; Achieve a common 6 oxygen atoms on either side of the. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). Al. What Is The Balanced Equation For Aluminum And Oxygen.
From www.slideserve.com
PPT Word equations to balanced chemical equations drill Each of the What Is The Balanced Equation For Aluminum And Oxygen The balanced equation will appear. The balanced chemical equation is: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This is the unbalanced equation described. Achieve a common 6 oxygen atoms on either side of the. In this problem, we need to write the balanced equation for a reaction between aluminium and. What Is The Balanced Equation For Aluminum And Oxygen.
From bernard-blogrose.blogspot.com
Aluminum Reacts With Oxygen to Produce Aluminum Oxide Chemical Equation What Is The Balanced Equation For Aluminum And Oxygen This is the unbalanced equation described. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Achieve a common 6 oxygen atoms on either side of the. In a reaction between aluminium and. The balanced equation will appear. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). Symbol for aluminium is al and. What Is The Balanced Equation For Aluminum And Oxygen.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas What Is The Balanced Equation For Aluminum And Oxygen Don't forget it's diatomic!) to produce aluminum oxide (al2o3). The balanced chemical equation for this. In a reaction between aluminium and. Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. The balanced equation will appear. Al + o2 → al2o3. In this problem, we need to write the balanced equation. What Is The Balanced Equation For Aluminum And Oxygen.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry What Is The Balanced Equation For Aluminum And Oxygen Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. The balanced chemical equation is: This is the unbalanced equation described. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Achieve a common 6. What Is The Balanced Equation For Aluminum And Oxygen.
From www.chegg.com
Solved The balanced equation for the reaction of aluminum What Is The Balanced Equation For Aluminum And Oxygen This is the unbalanced equation described. Achieve a common 6 oxygen atoms on either side of the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation for aluminum and oxygen is: In a reaction between aluminium and. In this problem, we need to write the balanced equation for a. What Is The Balanced Equation For Aluminum And Oxygen.
From www.youtube.com
How to Balance Al + O2 = Al2O3 YouTube What Is The Balanced Equation For Aluminum And Oxygen In a reaction between aluminium and. The balanced chemical equation for this. Al + o2 → al2o3. This balanced equation ensures that the number of atoms of each. The balanced chemical equation is: In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). Symbol. What Is The Balanced Equation For Aluminum And Oxygen.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive What Is The Balanced Equation For Aluminum And Oxygen The balanced chemical equation is: The balanced equation will appear. This is the unbalanced equation described. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. In a reaction between aluminium and. The balanced equation for aluminum and oxygen is: The balanced chemical equation for this. Al + o2 → al2o3. Achieve a. What Is The Balanced Equation For Aluminum And Oxygen.
From www.chegg.com
Solved Aluminum reacts with oxygen to produce aluminum oxide What Is The Balanced Equation For Aluminum And Oxygen This balanced equation ensures that the number of atoms of each. The balanced equation will appear. We're given that aluminum (al) can react with oxygen gas (o2; The balanced chemical equation is: The balanced equation for aluminum and oxygen is: Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. This. What Is The Balanced Equation For Aluminum And Oxygen.
From bernard-blogrose.blogspot.com
Aluminum Reacts With Oxygen to Produce Aluminum Oxide Chemical Equation What Is The Balanced Equation For Aluminum And Oxygen In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. This is the unbalanced equation described. In a reaction between aluminium and. The balanced equation for aluminum and oxygen is: Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. The balanced chemical equation. What Is The Balanced Equation For Aluminum And Oxygen.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas What Is The Balanced Equation For Aluminum And Oxygen The balanced equation will appear. The balanced chemical equation for this. Achieve a common 6 oxygen atoms on either side of the. Al + o2 → al2o3. This balanced equation ensures that the number of atoms of each. This is the unbalanced equation described. In this problem, we need to write the balanced equation for a reaction between aluminium and. What Is The Balanced Equation For Aluminum And Oxygen.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas What Is The Balanced Equation For Aluminum And Oxygen The balanced chemical equation is: In a reaction between aluminium and. The balanced equation for aluminum and oxygen is: We're given that aluminum (al) can react with oxygen gas (o2; Don't forget it's diatomic!) to produce aluminum oxide (al2o3). This balanced equation ensures that the number of atoms of each. The balanced equation will appear. 4al(s) +3o2(g) → 2al2o3(s) according. What Is The Balanced Equation For Aluminum And Oxygen.
From www.numerade.com
SOLVEDWrite a balanced equation to represent the electrolysis of What Is The Balanced Equation For Aluminum And Oxygen Don't forget it's diatomic!) to produce aluminum oxide (al2o3). To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This is the unbalanced equation described. We're given that aluminum (al) can react with oxygen gas (o2; The balanced equation will appear. 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al. What Is The Balanced Equation For Aluminum And Oxygen.
From www.numerade.com
SOLVED Aluminum metal reacts with oxygen gas to produce aluminum oxide What Is The Balanced Equation For Aluminum And Oxygen To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In a reaction between aluminium and. The balanced equation for aluminum and oxygen is: Achieve a common 6 oxygen atoms on either side of the. This balanced equation ensures that the number of atoms of each. Symbol for aluminium is al and it. What Is The Balanced Equation For Aluminum And Oxygen.
From www.numerade.com
SOLVED Write the balanced equation for the formation of aluminum oxide What Is The Balanced Equation For Aluminum And Oxygen In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Al + o2 → al2o3. This is the unbalanced equation described. 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3. What Is The Balanced Equation For Aluminum And Oxygen.
From www.youtube.com
Write a balanced equation for the reaction betwee aluminium oxide What Is The Balanced Equation For Aluminum And Oxygen The balanced chemical equation is: Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Al + o2 → al2o3. The balanced chemical equation for this. In a reaction between aluminium and. This balanced. What Is The Balanced Equation For Aluminum And Oxygen.
From www.researchgate.net
2 Oxygen balance equations. Download Scientific Diagram What Is The Balanced Equation For Aluminum And Oxygen 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. The balanced chemical equation is: The balanced equation will appear. In a reaction between aluminium and. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. We're given that aluminum (al) can react with oxygen gas. What Is The Balanced Equation For Aluminum And Oxygen.
From www.youtube.com
Writing the Formula for Aluminum Oxide YouTube What Is The Balanced Equation For Aluminum And Oxygen This balanced equation ensures that the number of atoms of each. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). The balanced equation for aluminum and oxygen is: In a reaction between aluminium and. The balanced chemical equation is: The balanced equation will appear. Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence). What Is The Balanced Equation For Aluminum And Oxygen.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas What Is The Balanced Equation For Aluminum And Oxygen To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. Al + o2 → al2o3. The balanced equation will appear. We're given that aluminum (al) can react with oxygen gas (o2; The balanced chemical equation for. What Is The Balanced Equation For Aluminum And Oxygen.
From www.numerade.com
SOLVED Consider the unbalanced equation for aluminum and oxygen What Is The Balanced Equation For Aluminum And Oxygen Achieve a common 6 oxygen atoms on either side of the. We're given that aluminum (al) can react with oxygen gas (o2; Al + o2 → al2o3. In a reaction between aluminium and. Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. 4al(s) +3o2(g) → 2al2o3(s) according to the equation,. What Is The Balanced Equation For Aluminum And Oxygen.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free What Is The Balanced Equation For Aluminum And Oxygen To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Achieve a common 6 oxygen atoms on either side of the. The balanced equation for aluminum and oxygen is: Al + o2 → al2o3. The balanced chemical equation for this. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). In a reaction between. What Is The Balanced Equation For Aluminum And Oxygen.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas What Is The Balanced Equation For Aluminum And Oxygen The balanced chemical equation is: 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. In a reaction between aluminium and. Al + o2 → al2o3. Don't forget it's diatomic!) to produce aluminum oxide (al2o3). Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its. What Is The Balanced Equation For Aluminum And Oxygen.
From www.onlinemathlearning.com
How to balance chemical equations (solutions, examples, videos) What Is The Balanced Equation For Aluminum And Oxygen Symbol for aluminium is al and it has +3 as its most stable oxidation state (valence) in its oxide. 4al(s) +3o2(g) → 2al2o3(s) according to the equation, 4 moles of al react with 3 moles of. This is the unbalanced equation described. In this problem, we need to write the balanced equation for a reaction between aluminium and oxygen. The. What Is The Balanced Equation For Aluminum And Oxygen.