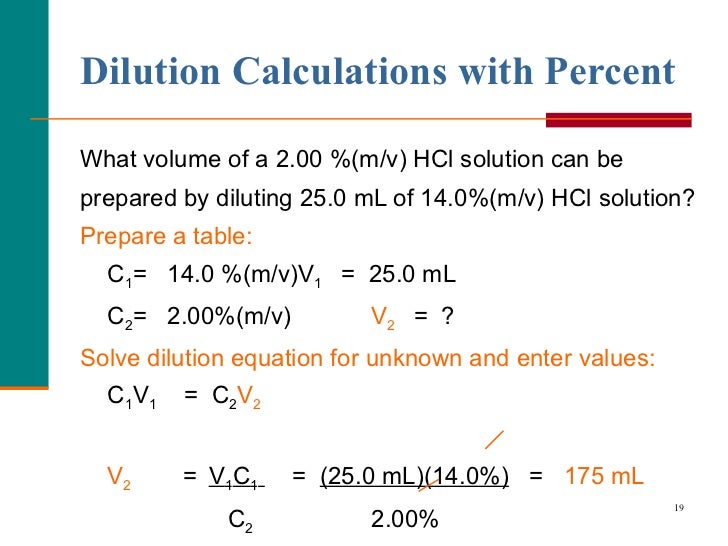
Dilution Concentration Equation . dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the following equation shows the relationship between concentration and volume before and after dilution. state whether the concentration of a solution is directly or indirectly proportional to its volume. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
from www.slideshare.net
dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the following equation shows the relationship between concentration and volume before and after dilution. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
Molarity and dilution
Dilution Concentration Equation dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Concentration Equation state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the. Dilution Concentration Equation.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution factor in Serial Dilution Concentration Equation Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the. Dilution Concentration Equation.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation ID543259 Dilution Concentration Equation the following equation shows the relationship between concentration and volume before and after dilution. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. solutions can be prepared. Dilution Concentration Equation.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free download ID1014345 Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. state whether the concentration. Dilution Concentration Equation.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Formula & Equation Stock Dilution Concentration Equation Concentration is the removal of solvent, which. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Concentration Equation.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry Dilution Concentration Equation Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. solutions can be prepared by diluting a more concentrated solution rather than starting with. Dilution Concentration Equation.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. the following equation shows the relationship between concentration and volume before and after dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the. Dilution Concentration Equation.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or. Dilution Concentration Equation.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. dilution is the addition. Dilution Concentration Equation.
From www.slideshare.net
Molarity and dilution Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. . Dilution Concentration Equation.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations YouTube Dilution Concentration Equation solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases. Dilution Concentration Equation.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Concentration Equation Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. the following equation shows the relationship between concentration and volume before and after dilution. dilution is. Dilution Concentration Equation.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. the following equation shows the relationship between. Dilution Concentration Equation.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the. Dilution Concentration Equation.
From www.youtube.com
Dilution Ventilation Controlling Contaminant Concentration Example 1 YouTube Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the following equation shows. Dilution Concentration Equation.
From www.slideshare.net
Molarity and dilution Dilution Concentration Equation state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with. Dilution Concentration Equation.
From www.youtube.com
CHEMISTRY 101 Calculating Ion Concentration by Molarity and Solution Dilution YouTube Dilution Concentration Equation Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following. Dilution Concentration Equation.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Concentration Equation solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. dilution is. Dilution Concentration Equation.
From www.slideserve.com
PPT Chapter 1 The Organization of Matter PowerPoint Presentation, free download ID5754096 Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. Concentration is the removal of solvent, which. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. state whether. Dilution Concentration Equation.
From dxohqxlgl.blob.core.windows.net
Dilution 1 Volume at Jeremiah Gonzalez blog Dilution Concentration Equation solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. . Dilution Concentration Equation.
From onthewebvil.weebly.com
Formula for concentration dilution onthewebvil Dilution Concentration Equation state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and. Dilution Concentration Equation.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID949751 Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. dilution is the process of decreasing the concentration of a. Dilution Concentration Equation.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID3874312 Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the process of decreasing the. Dilution Concentration Equation.
From www.pdfprof.com
how to calculate dilution factor Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. Concentration is the removal of solvent, which. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is. Dilution Concentration Equation.
From www.slideserve.com
PPT Chapter 1 The Organization of Matter PowerPoint Presentation, free download ID5754096 Dilution Concentration Equation dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the following equation shows the relationship between concentration and volume before and after dilution. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the addition of. Dilution Concentration Equation.
From ar.inspiredpencil.com
Dilution Equation Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. the following equation shows the relationship between concentration and volume before and after dilution. dilution is the process of decreasing the concentration of a solute. Dilution Concentration Equation.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. the following equation shows the relationship between concentration and volume before and after dilution. state whether. Dilution Concentration Equation.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Concentration Equation dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Concentration is the removal of solvent, which. the following equation shows the relationship between concentration and volume before and after dilution. state whether the concentration of a solution is directly or indirectly proportional to its volume. . Dilution Concentration Equation.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Concentration Equation Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by. Dilution Concentration Equation.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solutions can be prepared by diluting a more concentrated solution. Dilution Concentration Equation.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the addition of solvent, which decreases. Dilution Concentration Equation.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Concentration Equation the following equation shows the relationship between concentration and volume before and after dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process of decreasing the concentration of a solute. Dilution Concentration Equation.
From www.freepik.com
Premium Vector Dilution concentration formula science vector illustration graphic Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the. Dilution Concentration Equation.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Example 4 YouTube Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases. Dilution Concentration Equation.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Concentration Equation dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the following equation shows the relationship between concentration and volume before and after dilution. state whether the concentration of a solution is directly or. Dilution Concentration Equation.